Janssen
Aug 29, 2023
FDA Approves BMS’s Reblozyl for MDS; FDA Awards Orphan Drug Designation to NXC-201; Janssen Submits Supplemental NDA for Full Approval of BALVERSA; FDA Grants Fast Track Status to ALE.C04; FDA Orphan Drug Designation to Faron’s Bexmarilimab; FDA Clears IND Application for AHB-137
FDA Approves Bristol Myers Squibb’s Reblozyl as First-Line Treatment of Anemia in Adults with Lower-Risk MDS Who May Require Transfusions Bristol Myers Squibb announced that the Food and Drug Administration (FDA) has approved Reblozyl® (luspatercept-aamt) for the treatment of anemia in adult patients with very l...
Read More...
Aug 15, 2023
J&J’s 2-in-1 Tablet for Prostate Cancer; FDA Approves TALVEY for Heavily Pretreated Multiple Myeloma; PDS Biotech Updated on VERSATILE-003 Trial; FDA Issues CRL to NDA for Avasopasem in Radiotherapy-Induced Severe Oral Mucositis in HNC; FDA Orphan Drug Designation to Genprex’s REQORSA; FDA Orphan Drug Designation to Bloomsbury’s BGT-OTCD
FDA Clears J&J’s 2-in-1 Tablet for Prostate Cancer Johnson & Johnson's Janssen Pharmaceutical Companies stated that the US Food and Drug Administration (FDA) had approved AKEEGA (niraparib and abiraterone acetate), the first-and-only dual-action tablet combining a PARP inhibitor including abiraterone ace...
Read More...
Apr 25, 2023
Janssen’s AKEEGA Approval; FDA Approves Roche’s Polivy Combo for Frontline B-cell Lymphoma; Daiichi Sankyo’s Quizartinib for Adults With FLT3-ITD-Positive AML; bluebird bio BLA for lovo-cel for Patients with Sickle Cell Disease; Fast Track Designation for Lu-PNT2002 for mCRPC Treatment; FDA Orphan Drug Designation to XORTX’s Oxypurinol
Janssen Marks First Approval Worldwide for AKEEGA® (Niraparib and Abiraterone Acetate Dual Action Tablet) The Janssen Pharmaceutical Companies of Johnson & Johnson announced that the European Commission (EC) had granted marketing authorization for AKEEGA® (niraparib and abiraterone acetate [AA]), in the form...
Read More...
Dec 03, 2020
Roche’s quantitative COVID-19 antibody test; Janssen buys a gene therapy asset; Zimmer Acquires A&E Medical; Glaucoma research study: Merck chooses TriNKET cancer immunotherapy program; Ovid fails Angelman phase 3 study
Roche receives FDA authorization for the quantitative COVID-19 antibody test Roche has got an authorization from the FDA for a more accurate COVID-19 blood test capable of measuring the levels of specific antibodies, which target the cell-unlocking spike protein of coronavirus. The company anticipates the tes...
Read More...
Jul 02, 2019
FDA’s approval to Pfizer’s Zirabev; Launch of Century Therapeutics; Imbruvica’s recommendation from CHMP
Pfizer’s Zirabev gets FDA nod for the treatment of five types of cancer The US FDA has approved Zirabev (bevacizumab-bvzr), a drug developed by Pfizer, for the treatment of five different types of cancer namely metastatic colorectal cancer; unresectable, locally advanced, recurrent or metastatic non-squamous no...
Read More...
Oct 11, 2018
Copiktra receives approval; Lilly wins approval; Eisai’s Fycompa; Astellas’ Roxadustat; Janssen’s Esketamine Nasal Spray
SNAPSHOTS: Copiktra receives FDA approval for CLL and FL, Relief for tazemetostat developers after FDA lifted partial clinical hold, Conference highlights from 19th WCLC The United States Food and Drug Administration (USFDA) has granted approval to Verastem’s Copiktra (duvelisib), for the treatment of patients with...
Read More...
Oct 11, 2018
Orchard files for IPO; J&J puts money on RNA interference; Rgenix receives $40M; GSK gives a helping hand
Orchard files for USD173 Million IPO to run pivotal gene therapy trials Orchard Therapeutics, Transatlantic biotech, has filed to raise USD 173 million in an IPO. This will lead it to take three gene therapies through the clinic. Orchard recognised itself as one of the broadest clinical-phase gene therapy pipelines ...
Read More...
Oct 04, 2018
Hologic complements Focal; Allogene for IPO; Gamida, Novartis-backed cell therapy startup; Aduro loses partner
Hologic complements Focal Therapeutics to its breast-conserving surgery for USD 125 Million Hologic, a global champion of women's health based in Massachusetts, United States, is taking possession of Focal Therapeutics, a medical device company, for USD 125 million to complement its breast-conserving surgery permit...
Read More...
Jun 22, 2018
Fatty Acid Amide Hydrolase (FAAH) Inhibitor – an enzyme with novel therapeutic potential
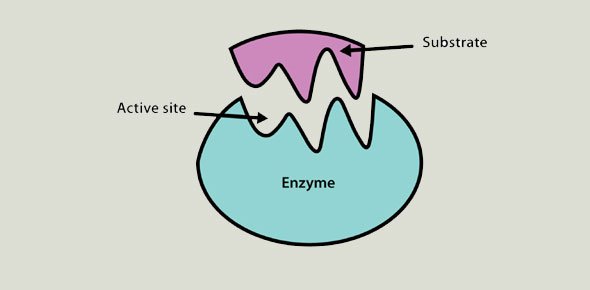
Fatty acid amide hydrolase (FAAH) belongs to the serine hydrolase family of enzymes. The identification of this enzyme was led by the discovery and characterization of fatty acid amides, including anandamide and oleamide, as a fundamental class of endogenous signaling molecules. FAAH serves as the major metabolic re...
Read More...
Oct 04, 2016
FDA Approves STELARA; Novartis announces AMG 334; AbbVie’s HCV Regimen; PaizaBio Gains CFDA Approval; WuXi Biologics completes construction
FDA Approves Janssen's STELARA for the Treatment of Adults With Moderately to Severely Active Crohn’s Disease Janssen Biotech Inc. received approval from the U.S. FDA for STELARA (ustekinumab), used for the treatment of moderately to severely active Crohn’s disease in adults (18 years or older). The drug is for the ...
Read More...







-Agonist.png)

