Johnson and Johnson
Nov 29, 2024
Novel Mutation-Targeting Therapies in the Horizon to Relieve the Global Healthcare Burden NSCLC Poses
Lung cancer, to date, remains the leading cause of death worldwide, however, the epidemiological analysis depicts varying NSCLC incidence all over the world. In 2022, there were an estimated 20 million new cancer cases and 9.7 million cancer-related deaths. The number of people living for at least five years after ...
Read More...
Feb 22, 2024
Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better Therapeutics’s MASH Treatment; X-trodes’ Wearable “Skin” Solution; Lumicell’s LUMISIGHTTM; Konesksa Enrolled First Patient in Learns Observational Study
Johnson & Johnson, Launched New Intraocular Lens in EMEA Region On February 15, 2024, Johnson & Johnson Medtech’s vision unit launched the Tecnis PureSee lens in the EMEA region. Tecnis PureSee is a purely refractive intraocular lens (IOL) specifically designed to correct presbyopia. Its proprietary desi...
Read More...
Feb 08, 2024
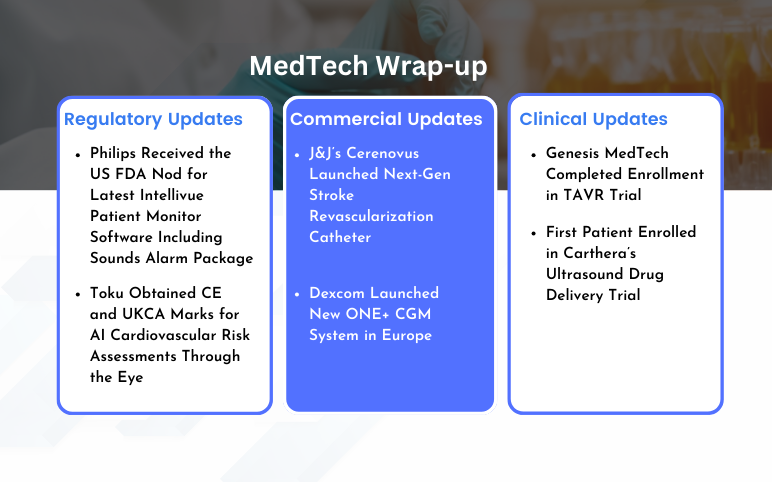
Cerenovus Launched Next-Gen Stroke Revascularization Catheter; Dexcom Launched New ONE+ CGM System; Philips Received the US FDA Nod for Latest Intellivue Patient Monitor Software; Toku Obtained CE and UKCA Marks for AI Cardiovascular Risk Assessments; Genesis MedTech Completed Enrollment in TAVR Trial; Carthera’s Ultrasound Drug Delivery Trial
J&J’s Cerenovus Launched Next-Gen Stroke Revascularization Catheter On February 7, 2024, Johnson & Johnson MedTech’s Cerenovus announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse indicated for the revascularization of patients suffering from acute ischemic stroke...
Read More...
Jan 17, 2024
A Glance at Key Insights From 42nd J.P. Morgan Annual Healthcare Conclave
From January 8th to 11th, 2024, the 42nd Annual J.P. Morgan Healthcare Conference (JPM24) took center stage in San Francisco, CA, USA. Spanning four dynamic days, this conference saw the active participation of prominent figures from major pharmaceutical, biotechnology, Medtech, HealthTech entities, and emerging fa...
Read More...
Dec 21, 2023
Integra to Buy J&J’s Acclarent; B. Braun Launches the CARESITE Micro Luer Access Devic; FDA Approves Medtronic’s Novel PulseSelect Pulsed Field Ablation System; FDA Clearances to Perfuze’s Novel Neurovascular Aspiration and Access Catheters for Stroke Treatment; ShiraTronics Successfully Implants First Migraine System; Sight Sciences Announced the Six-Month Results For Heat Therapy Device
Integra LifeSciences to Buy Johnson & Johnson’s Acclarent and its ENT Tech On December 13, 2023, Integra LifeSciences entered into a definitive agreement to acquire Acclarent from Johnson & Johnson MedTech. Acclarent strengthens Integra's position in the ENT treatment market. Acclarent is a component of ...
Read More...
Dec 07, 2023
Johnson & Johnson Medtech Acquired Laminar; BD Launched Advanced Vascular Access Ultrasound System; FDA Cleared Oral Device for Severe Sleep Apnea; FDA Clearanced the Zilia OcularTM FC Retinal Camera; First Patient Enrolled in Penumbra Study of Computer-assisted Vacuum Thrombectomy; US FDA Granted the BiVACOR Total Artificial Heart IDE Approval for First-in-Human Early Feasibility Study
FDA Cleared Oral Device for Severe Sleep Apnea From Vivos Therapeutics On November 29, 2023, Vivos Therapeutics announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Vivos’s removable CARE (Complete Airway Repositioning and Expansion) oral appliances developed for treat...
Read More...
Dec 01, 2023
Could Amgen’s Biosimilar Wezlana Pose a Challenge to Johnson & Johnson’s Stelara
The FDA recently approved Amgen’s Wezlana (ustekinumab-auub) for various inflammatory conditions, marking the first biosimilar approval referencing the popular J&J drug Stelara (ustekinumab). Wezlana, mirroring its reference product, is approved for treating multiple inflammatory diseases in adults, including m...
Read More...
Nov 23, 2023
Ethicon’s ETHIZIA Hemostatic Sealing Patch; FDA Approves Medtronic’s Minimally Invasive Device to Treat Hypertension; Boston Scientific Acquires Relievant Medsystems; AstraZeneca Launched Evinova; Surmodics Announced Data of the SWING Trial; GSK Advancing the Low Carbon Ventolin Program to Phase III Trials
Ethicon Announced European Approval and Introduction of ETHIZIA™ Hemostatic Sealing Patch Used to Stop Disruptive Bleeding On November 15, 2023, Ethicon, a Johnson & Johnson MedTech company, received approval for ETHIZIA™, an adjunctive hemostat solution that has been clinically proven to achieve sustained h...
Read More...
Nov 16, 2023
GE Healthcare and Masimo Announced Collaboration; Samsung Launched New Ultrasound System ‘V6’; MONARCH Platform Approved for Bronchoscopy in China; Lunit Received FDA Nod for Lunit INSIGHT DBT; Route 92 Medical Releases Tenzing Technology Clinical Findings; Vivasure Medical Enrolled 100th Patient for its Vivasure PerQseal Closure Device System Study
GE HealthCare and Masimo Collaborated on Patient Monitoring Tech On November 8, 2023, GE HealthCare and Masimo announced a joint agreement to integrate patient monitoring technologies. The goal of the partnership is to incorporate Masimo Signal Extraction Technology (SET) pulse oximetry into GE HealthCa...
Read More...
Aug 31, 2023
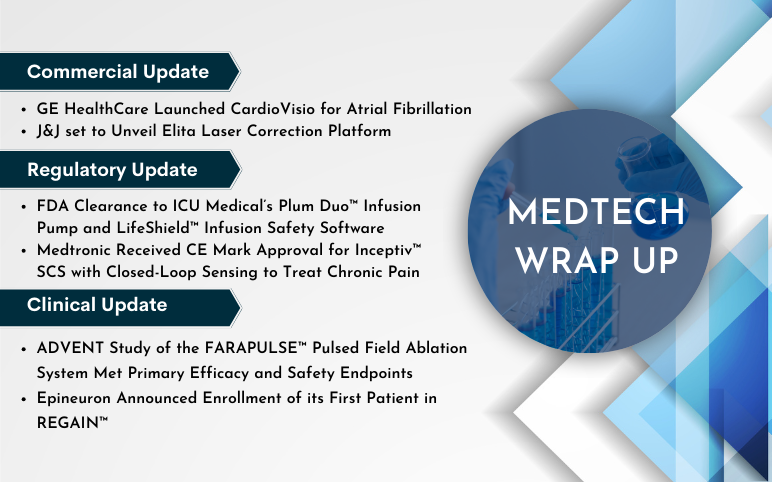
GE HealthCare Launched CardioVisio; J&J’s Elita Laser Correction Platform; ICU Medical’s Plum Duo Infusion Pump and LifeShield Infusion Safety Software Update; Medtronic’s Inceptiv SCS with Closed-Loop Sensing to Treat Chronic Pain; Boston Scientific’s FARAPULSE Pulsed Field Ablation System; Epineuron’s REGAIN Trial
GE HealthCare Launched CardioVisio for Atrial Fibrillation, a Digital, Patient-Centric Clinical Decision Support Tool to Enable Precision Care On August 24, 2023, GE HealthCare launched CardioVisio for Atrial Fibrillation (AFib), a digital tool designed to assist clinicians in visualizing longitudinal data relev...
Read More...






-Agonist.png)

