Magnetic Resonance Imaging Devices Market
May 12, 2022
Abbott’s Alinity m STI Assay; Phillips’ MR 7700 System; HOYA’s MiYOSMART Spectacle Lens; Magneto’s Pulmonary Embolism Treatment; Owen Mumford and Stevanto Group’s Collaboration Deal; Elekta’s Radiosurgery System-Elekta Esprit
Abbott Receives Regulatory Approval from US FDA for Alinity™ m STI Assay On May 04, 2022, the US Food and Drug Administration (FDA) granted the regulatory approval to Alinity™ m STI Assay developed by Abbott which is capable of simultaneously detecting and differentiating four common sexually trans...
Read More...
Apr 07, 2022
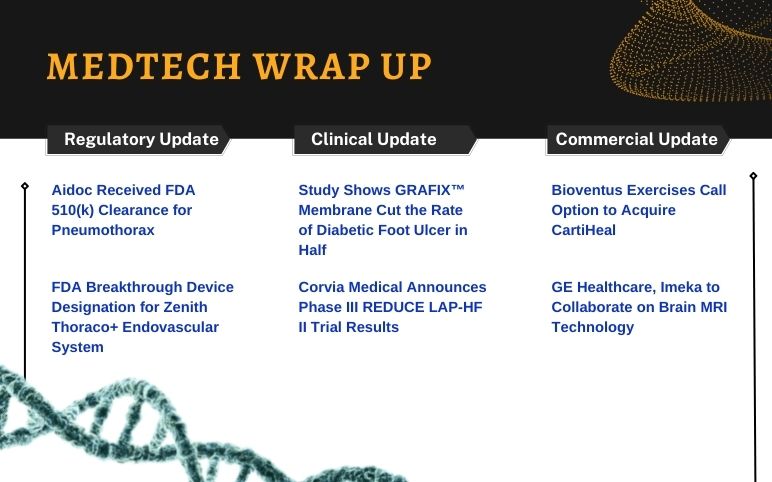
Aidoc’s Pneumothorax; Cook Medical’s Zenith Thoraco+ Endovascular System; GRAFIX Membrane Study; Corvia’s REDUCE LAP-HF II Trial Results; GE Healthcare and Imeka’s Collaboration; Bioventus to Acquire CartiHeal
Aidoc Expands AI Service to X-ray, Receiving FDA 510(k) Clearance for Pneumothorax On March 30, 2022, Aidoc, the leading developer of healthcare AI solutions, announced that its triage and notification of pneumothorax on X-ray exams has gained FDA 510(k) approval. Aidoc's other seven FDA-cleared clinical AI prod...
Read More...

-Agonist.png)

