medical device
Jul 04, 2024
Artivion and Endospan Agreement; Masimo and Cleveland Clinic Collaboration; Academic Medical Center’s Sedation-Free TNE With the EvoEndo Endoscopy System; IceCure’s Next-Gen XSenseTM Cryoablation System FDA Approval; KORU Medical Systems’ Clearance for FreedomEdge® Infusion System; Reflow Medical’s First Patient in Coronary Sirolimus-Eluting Retrievable Scaffold System
Artivion Amended Agreements With Endospan On June 01, 2024, Artivion, Inc. a prominent company in cardiac and vascular surgery specializing in aortic disease, announced that it revised its credit facility and option purchase agreements with Endospan Ltd. ("Endospan"), an Israeli-based, privately-held developer o...
Read More...
Jun 27, 2024
Alcon’s 510(k) Clearance From the FDA; Globus Medical Received FDA Clearance; UK Pioneers First Skull-Mounted Epilepsy Device for Child; The Initiation of a Significant Clinical Trial of 3d Printed Models; Esaote Unveiled the Cutting-Edge Mylab™E80 @Sirm Ultrasound Device For 2024; Medasense Unveiled a Transformative Partnership With Nihon Kohden Corporation
Alcon's Newest Technological Marvels, Unity VCS, and Unity CS, Achieved 510(k) Clearance From the U.S. FDA, Paving the Way for New Advancements On June 24, 2024, Alcon, the foremost name in eye care with a mission to help people see brilliantly, revealed that the U.S. Food and Drug Administration (FDA) had...
Read More...
Jun 20, 2024
Boston Scientific Corporation Agreement to Acquire Silk Road Medical; Philips Introduced Duo Venous Stent System; Akili’s EndeavorOTC Received FDA Clearance; Roche Achieved FDA Clearance; atHeart Medical Reported Long and Short-term Clinical Data; Bioretec Announced Successful Clinical Outcomes
Boston Scientific Corporation Announced Definitive Agreement to Acquire Silk Road Medical, Inc., an Innovator in Stroke Prevention Technology On June 18, 2024, Boston Scientific Corporation, a global leader in medical technologies, announced the formal agreement to acquire Silk Medical, Inc., a medical device co...
Read More...
Jun 13, 2024
Asensus Surgical Acquired by Karl Storz; Allosource® Launched Aceconnex®; Siemens Healthineers FDA Clearance for Chiller-Free Biograph Trinion PET/CT; Abbott Received FDA Clearance; Foldax® Reported Positive Clinical Results; Amber Implants Completed Enrolment of First-In-Human Clinical Trial
Asensus Surgical Agreed to be Acquired by Karl Storz On June 07, 2024, Asensus Surgical announced that it had entered a deal in which Karl Storz would acquire the company for 35¢ per share in cash. Karl Storz Endoscopy-America, a wholly-owned subsidiary of Germany-based Karl Storz, had agreed to acquire...
Read More...
Jun 06, 2024
Stryker’s LIFEPAK 35 Monitor/Defibrillator; Qiagen’s QIAstat-Dx Respiratory Panel; Evolution Optiks’s 510(k) Clearance; Microbot Medical Endovascular Surgical Robot Trial; Nexalin’s Insomnia and Anxiety Therapy Headset Clinical Trial; KORU Medical Systems’ Study With Commercialized Oncology Biologics
Stryker Released LIFEPAK 35 Monitor/Defibrillator On June 04, 2024, Stryker, a global leader in medical technologies, announced the launch of the LIFEPAK 35 monitor/defibrillator. This latest addition to their monitor/defibrillator lineup features advanced technology and is designed on an intuitive, modern...
Read More...
May 30, 2024
Solis Mammography’s Acquisition of MUSIC Imaging Center; Nikon’s SI-PH Phase Condenser Accessory Option; Teijin Launch Surgical Patch; Terumo Cardiovascular’s FDA 510(K) Clearance; Spineart’s 510(k) Clearance; Ceretrieve’s Success in FIH Ischemic Stroke Cases
Solis Mammography Announced Acquisition of MUSIC Imaging Center in Gainesville, Florida On May 28, 2024, Solis Mammography announced the acquisition of the MUSIC Mammography and Ultrasound Imaging Center in Gainesville, Florida. For more than 10 years, MUSIC has provided patients with an advanced standard ...
Read More...
May 23, 2024
Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; Implicity’s Heart Failure Algorithm FDA 510(k) Approval; AngioDynamics’ AlphaVac F1885 System CE Mark Approval; HeartBeam’s Artificial Intelligence Algorithm for Arrhythmias; Onward ARC-EX Nerve Stim Hit Pivotal Trial
Laborie Launched Solar Compact System and Solar Anorectal Manometry Catheter for Enhanced Gastrointestinal Diagnostics On May 20, 2024, Laborie Medical Technologies Corp., launched the Solar Compact System and Solar Anorectal Manometry Catheter, the first disposable HRAM catheter on the market. These devices sig...
Read More...
May 16, 2024
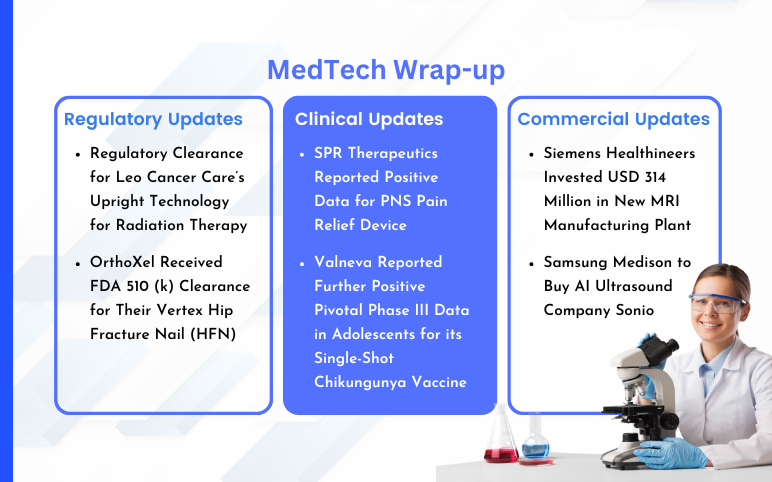
Siemens Healthineers USD 314 Million Investment; Samsung Medison’s Sonio Acquisition; Leo Cancer Care’s Upright Technology Clearance; OrthoXel’s Vertex Hip Fracture Nail FDA 510 (k) Clearance; SPR Therapeutics’ PNS Pain Relief Device Positive Data; Valneva’s Chikungunya Vaccine Positive Pivotal Phase III Data
Siemens Healthineers Invested USD 314 Million in New MRI Manufacturing Plant On May 15, 2024, Siemens Healthineers announced that it had invested USD 314 million in a new MRI manufacturing plant in the UK. The facility in Oxford, England, which Siemens anticipates opening in 2026, will develop technology to redu...
Read More...
May 09, 2024
Monteris Smallest Brain Laser Probe Launch; Vesica Health’s AssureMDx Test Launch; Anaut’s Eureka α Japanese Regulatory Approval; Geneoscopy’s Labcorp-Partnered Colon Cancer Test FDA Approval; Novel Omeza® OCM™ Diabetic Foot Ulcers Results; LEO Pharma Phase III Plaque Psoriasis Trial Results
Monteris Launched Smallest Brain Laser Probe on the Market On May 01, 2024, Monteris Medical launched the NeuroBlate® NB3™ FullFire® 1.6mm laser probe, the company’s latest product line innovation for use with their market-leading NeuroBlate System. The NB3 laser probe, which integrates Monteris' patented coo...
Read More...
May 02, 2024
Xtant Medical’s SimipliGraft TM and SimpliMaxTM Launch; Perelel’s Fertility Support Products Expansion; Medtronic’s InceptivTM FDA Approval; GE Healthcare’s Vital Signs Monitor FDA 510(K) Clearance; Merck’s Pneumonia Vaccine Positive Results; Artivion’s Aortic Devices AATS Win
Xtant Medical Announced the Launch of SimipliGraft TM and SimpliMaxTM for Chronic and Acute Wounds On April 30, 2024, Xtant Medical Holdings, Inc., a global medical technology firm specializing in surgical remedies for spinal disorders, announced the complete commercial availability of two amniotic membran...
Read More...





-Agonist.png)

