medical device
Apr 25, 2024
iVEAcare’s $27.5 Million Series A Funding; AI Medical Service Joint Research Agreement with Mahidol University; CERENOVUS Launched Catheter; Cochlear FDA Clearance for The Osia System; Immunovia Announced Positive Results From The Model-Development Study; Hyalex Orthopaedics First Patients Treatment
iVEAcare Launched $27.5 Million Series A Funding from Leading Medtech Investors On April 24, 2024, iVEAcare, announced the closure of a $27.5 million Series A financing. The financing was led by Vensana Capital, which was joined by Treo Ventures, Hatteras Venture Partners, and an undisclosed strategic partner. i...
Read More...
Apr 18, 2024
Baxter Introduced New Injectable Pharmaceuticals to US Market; Bayer and Hologic Collaborated to Deliver Contrast-Enhanced Mammography; Labcorp Received FDA Emergency Use Authorization for Mpox PCR Test Home Collection Kit; Onkos Surgical Won FDA De Novo Nod For Antibacterial-Coated Orthopedic Implants; Heartbeam Presented Positive Results On Its Artificial Intelligence Capabilities For Detecting Arrhythmias; Cognito Therapeutics Showed Positive Non-Invasive Neuromod Results
Baxter Introduced New Injectable Pharmaceuticals to US Market On April 11, 2024, the pharmaceutical portfolio of multinational medical goods corporation Baxter International Inc. was augmented with the introduction of five new injectable medications in the US market. The goods were made to fulfill essential dema...
Read More...
Apr 11, 2024
Carl Zeiss Meditec AG’s Acquisition of Dutch Ophthalmic Research Center; Johnson & Johnson’s Shockwave Medical Acquisition; Biora Therapeutics’ BT-600 Positive Results; Medtronic’s Single-Shot Mapping Ablation Catheter Positive Data; Abbott’s TriClip FDA Approval; Abbott’s Whole Blood Rapid Test FDA Clearance
Carl Zeiss Meditec AG Completed Acquisition of Dutch Ophthalmic Research Center (D.O.R.C); Companies Unite to Shape Ophthalmology Market On April 04, 2024, Carl Zeiss Meditec AG announced that it acquired D.O.R.C. (Dutch Ophthalmic Research Center) from the investment firm Eurazeo SE, Paris, France. T...
Read More...
Apr 04, 2024
C2Dx’s Shaw Scalpel System; Texas Original’s RSO Gummies; Abbott’s Whole Blood Rapid Test FDA Clearance; Medtronic’s TAVR Device FDA Clearance; Nexalin’s Device Improved Symptoms; CYduct Diagnostics’ Positive Study Results
C2Dx Launched Next Generation Shaw Scalpel System On March 26, 2024, C2Dx, a privately-owned medical device company, announced the launch of its Next Generation Shaw Scalpel System. The Shaw Scalpel System has obtained 510(k) clearance from the FDA for the upgraded controller of its advanced surgical techn...
Read More...
Mar 14, 2024
Allumiqs and Prolytix’s Partnership; Setpoint Medical’s Neuroimmune Modulation Platform gets FDA Breakthrough Designation; MangoRx Launches ‘PRIME’ with FDA-approved TRT; SeaStar Medical Updates Quelimmune Commercial Launch; Ventris Medical Gains 510(k) for Amplify® Bone Graft Putty
MangoRx Officially Launches ‘PRIME’ by MangoRx, Powered by Kyzatrex®️ FDA Approved Oral Testosterone Replacement Therapy (TRT) Treatment On March 12, 2024, Mangoceuticals, Inc. unveiled a groundbreaking development eagerly awaited by many: the launch of 'PRIME' by MangoRx, Powered by Kyzatrex®️. This release mar...
Read More...
Mar 07, 2024
Philips Teamed with SyntheticMR; BioPhotas’s Celluma Light Therapy; Boston Scientific’s AGENTTM Drug-Coated Balloon; Varian’s TrueBeam and Edge Radiotherapy Systems; Carthera’s Brain-Blood Barrier Trial; Prototype Device Effectively Treated Multiorgan Failure
Philips Teamed with SyntheticMR to Deliver Breakthrough AI-based Quantitative Brain Imaging in MR to Advance Neurology Care For Patients On March 01, 2024, Philips in collaboration with SyntheticMR announced the launch of Smart Quant Neuro 3D, a significant advancement in objective decision support for diag...
Read More...
Feb 28, 2024
Navigating the Evolving Landscape of Pharmacy Automation Solutions
Pharmacy Automation Systems represent a pivotal advancement in healthcare technology, revolutionizing the way medications are dispensed, managed, and administered. Over the years, these systems have evolved from rudimentary pill counters to sophisticated robotic dispensers and integrated software platforms. Moreove...
Read More...
Feb 22, 2024
Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better Therapeutics’s MASH Treatment; X-trodes’ Wearable “Skin” Solution; Lumicell’s LUMISIGHTTM; Konesksa Enrolled First Patient in Learns Observational Study
Johnson & Johnson, Launched New Intraocular Lens in EMEA Region On February 15, 2024, Johnson & Johnson Medtech’s vision unit launched the Tecnis PureSee lens in the EMEA region. Tecnis PureSee is a purely refractive intraocular lens (IOL) specifically designed to correct presbyopia. Its proprietary desi...
Read More...
Feb 15, 2024
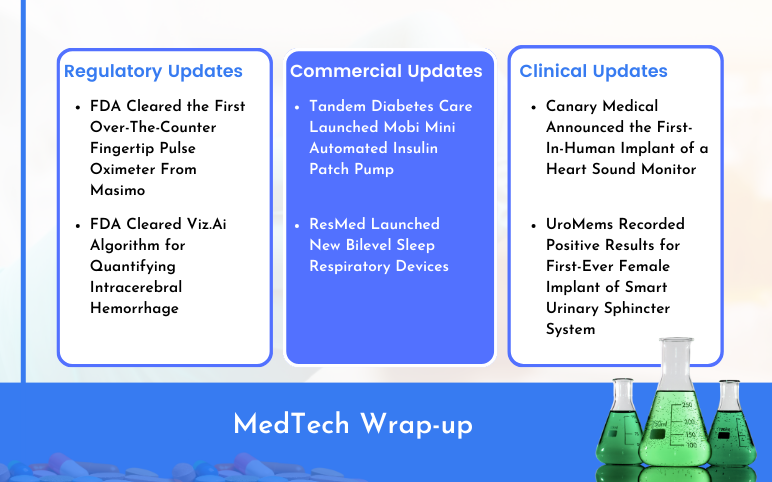
Tandem’s Automated Insulin Patch Pump; ResMed’s Bilevel Sleep Respiratory Devices; Masimo’s First Over-The-Counter Fingertip Pulse Oximeter; Viz.Ai Algorithm for Quantifying Intracerebral Hemorrhage; Canary Medical’s Heart Sound Monitor; UroMems’ Smart Urinary Sphincter System
Tandem Diabetes Care Launched Mobi Mini Automated Insulin Patch Pump On February 13, 2024, announced that it started off the U.S. commercial launch of its Mobi insulin patch pump. The San Diego-based business claims that Mobi, which is fully controllable via a smartphone app, is the world's smallest durable a...
Read More...
Feb 08, 2024
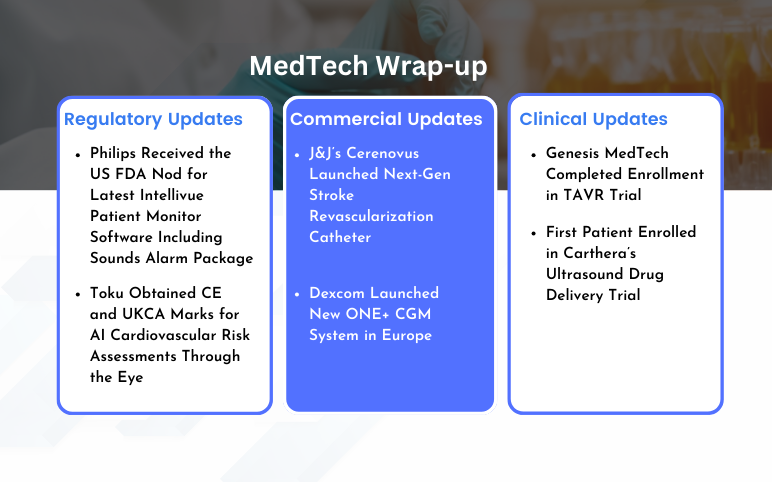
Cerenovus Launched Next-Gen Stroke Revascularization Catheter; Dexcom Launched New ONE+ CGM System; Philips Received the US FDA Nod for Latest Intellivue Patient Monitor Software; Toku Obtained CE and UKCA Marks for AI Cardiovascular Risk Assessments; Genesis MedTech Completed Enrollment in TAVR Trial; Carthera’s Ultrasound Drug Delivery Trial
J&J’s Cerenovus Launched Next-Gen Stroke Revascularization Catheter On February 7, 2024, Johnson & Johnson MedTech’s Cerenovus announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse indicated for the revascularization of patients suffering from acute ischemic stroke...
Read More...




-Agonist.png)

