MedTech Market
Oct 20, 2022
Biosense Webster’s HELIOSTAR Radiofrequency Balloon Ablation Catheter; Philips’s ClarifEye Augmented Reality Surgical Navigation Solution; NeuroLogica’s Elite Mobile Computed Tomography Devices; Medtronic’s Natural Conduction System for Heart; Pfizer & BioNTech’s Omicron BA.4/BA.5-Adapted Bivalent Booster Trial; Castle Biosciences’s TissueCypher® Barrett’s Esophagus Test
HELIOSTAR™ Radiofrequency Balloon Ablation Catheter Launched by Biosense Webster in Europe On October 12, 2022, Biosense Webster, a part of Johnson & Johnson Medical technology, announced the launch of HELIOSTAR™ Balloon Ablation Catheter, the first radiofrequency balloon ablation catheter in E...
Read More...
Oct 13, 2022
Neuspera Medical’s Nuvella System; Abbott Presented the Data for FreeStyle Libre 2 System; ZEISS Receives FDA 510(k) Clearance for MTLawton; FDA 510(k) Clearance for the GHOST 3D-Printed Titanium Spacer System; Thermo Fisher Scientific Launched CE-IVD Marked TaqPath Enteric Bacterial Select Panel; BIOCORP and Merck Announced New Partnership
Neuspera Medical Announces First Successful Implant of the Nuvella™ System in the Second Phase of Its Sans-Uui Ide Clinical Trial On October 10, 2022, Neuspera Medical, a medical device company involved in the development of implantable devices for patients suffering from chronic illnesses, announced that...
Read More...
Oct 12, 2022
Insights Into the Ongoing Developments and Key Trends in the Robotic Prosthetics Market
A prosthetic device or prosthesis is a great tool and plays a crucial role in the life of disabled people. Prosthetics help to perform nearly all their daily activities, such as walking, eating, dressing, and many more. These artificial limbs replace missing body parts and help function in nearly all the activities...
Read More...
Sep 29, 2022
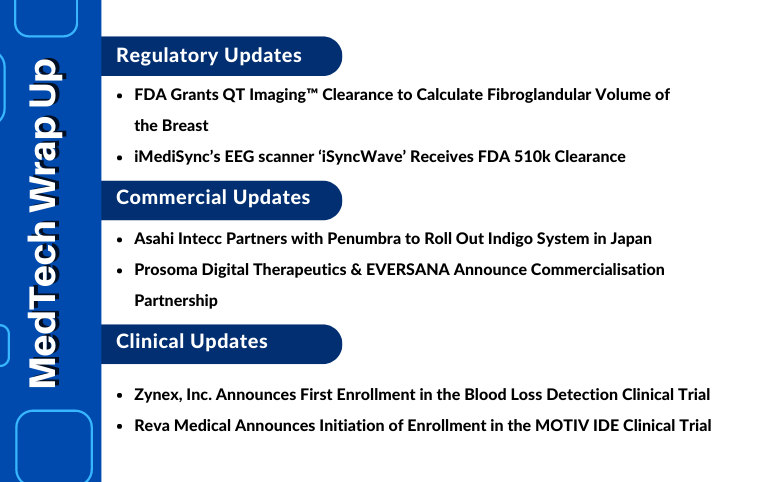
Asahi Intecc Partners with Penumbra; Prosoma and EVERSANA Announce Commercialisation Partnership; Zynex Starts Enrollment in the Blood Loss Detection Clinical Trial; Reva Initiates Enrollment in the MOTIV IDE Clinical Trial; FDA Clearance to QT Imaging to Calculate Fibroglandular Volume of the Breast; FDA 510k Clearance to iMediSync’s EEG scanner ‘iSyncWave’
Asahi Intecc partners with Penumbra to roll out Indigo System in Japan On September 22, 2022, Asahi Intecc, a Surgical and medical instrument manufacturing company, partnered with Penumbra, a medical device company headquartered in Alameda, California, to roll out Indigo System in Japan. After getting regulat...
Read More...
Sep 22, 2022
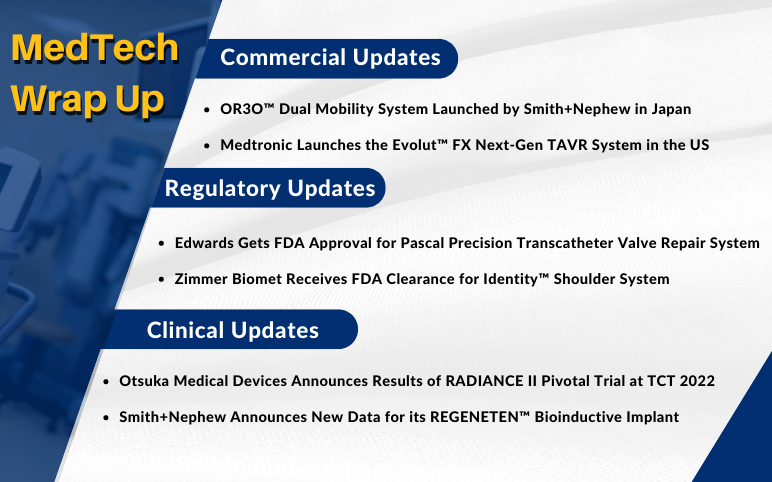
Smith+Nephew’s OR3O Dual Mobility System; Medtronic Launches the Evolut FX Next-Gen TAVR System; FDA Approves Edwards’s Pascal Precision Transcatheter Valve Repair System; FDA Clearance to Zimmer Biomet’s Identity Shoulder System; Otsuka Announces Results of RADIANCE II Trial; Smith+Nephew Announces Data for its REGENETEN™ Bioinductive Implant
OR3O™ Dual Mobility System launched by Smith+Nephew in Japan for use in primary and revision hip arthroplasty On September 20, 2022, Smith+Nephew, a leading global portfolio medical technology business, announced the launch of OR3O Dual Mobility System for use in primary and revision hip arthro...
Read More...
Sep 21, 2022
What Compelling Applications is Brain-computer Interfacing Bringing to the Healthcare Market?
Technological advancement is bringing in a new frontier in the healthcare segment with innovative tools and resourceful applications. With each passing day, it unlocks new mysteries and scales greater heights toward solving several underlying challenges. Now moving forward, how fascinating it will be if someone tel...
Read More...
Sep 15, 2022
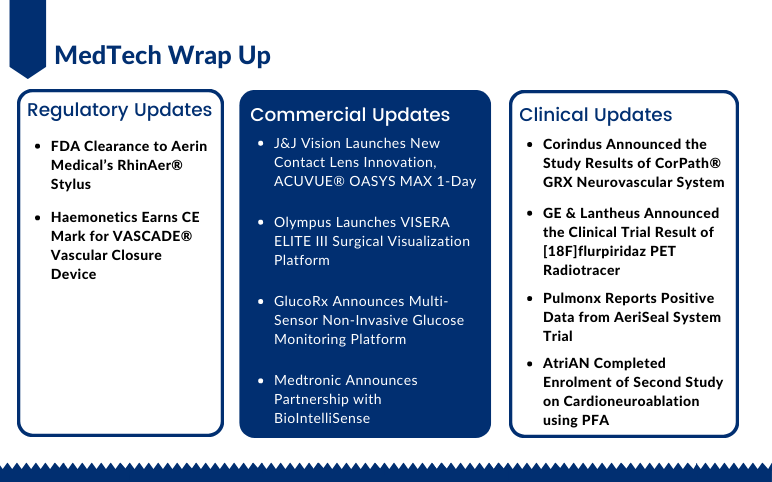
Aerin Medical’s RhinAer Stylus; CE Mark for Haemonetics’s VASCADE Vascular Closure Device; J&J Vision Launches ACUVUE OASYS MAX 1-Day; Olympus Launches VISERA ELITE III Surgical Visualization Platform; GlucoRx’s Non-Invasive Glucose Monitoring Platform; Medtronic-BioIntelliSense’s Partnership; Corindus’s CorPath GRX Neurovascular System Trial; GE & Lantheus [18F]flurpiridaz PET Radiotracer Trial; Pulmonx’s AeriSeal System Trial; AtriAN’s Second Study on Cardioneuroablation
Johnson & Johnson Vision Launches New Contact Lens Innovation ACUVUE® OASYS MAX 1-Day for Meeting the Needs of Digitally Intense Lifestyles On September 12, 2022, Johnson & Johnson Vision, a part of Johnson & Johnson and a global leader in the eyecare market, had announced the launch of its newest in...
Read More...
Sep 08, 2022
eCential Robotics’s Surgical Robotic Platform for Spine Surgery; Baxter Gets 510(k) Clearance for Syringe Pump; Medical Microinstruments’s NanoWrist Instruments; Magnus’ SAINT Neuromodulation System; Henry Schein Acquires Midway Dental Supply
eCential Robotics Receives FDA Clearance for its Surgical Robotic Platform for Spine Surgery The FDA has approved a robotic spinal surgery platform designed to assist human surgeons by automating several steps of spinal procedures. The platform, developed by eCential Robotics, combines intraoperative 2D and 3D i...
Read More...
Sep 07, 2022
How Is Digital Therapeutics Reshaping the Future of Healthcare?
Over the past few years, there has been a massive advancement and adoption in the digital health segment. The segment is likely to exhibit growth and onward trend in the coming years as well, owing to the huge capital investment and positive approach by the healthcare authorities Similar, some key factors, such as ...
Read More...
Sep 01, 2022
Miracor Medical’s Picso Pivotal Study; Medtronic’s Extravascular ICD; Galaxy Medical’s CENTAURI Pulsed Electric Field System; UltraSight’s Novel Cardiac AI Technology; Oticon Launched the World’s Smallest Hearing Aid; Medtronic Completes Acquisition of Affera
FDA Approved IDE for Picso® Pivotal Study of Miracor Medical On August 23, 2022, Miracor Medical SA, a provider of innovative solutions for the treatment of severe cardiac diseases, announced that the FDA has approved an Investigational Device Exemption (IDE), allowing the company to begin a pivotal study with i...
Read More...






-Agonist.png)

