MedTech Market
Jul 20, 2022
How are Nanobots Proving Their Excellence in the Healthcare Industry?
It is estimated that around five billion people are on the verge of losing access to essential healthcare services by 2030, for example, mandatory healthcare services, necessary medicine, and visiting healthcare providers. This issue can further become complex if there is an increase in the shortage of trained heal...
Read More...
Jun 29, 2022
What Impact Does Speech and Voice Recognition Technology Bring to the Healthcare System?
A software program and a hardware device that is capable of decoding a human voice is known as Voice recognition technology or Voice search technology. Voice search technology converts a spoken word into a command or text entry. Voice search is not just limited to recognizing the spoken word, it includes identifica...
Read More...
Jun 23, 2022
CE Mark to Ibex’s Gastric Cancer Detection System; Senseonics’s Eversense E3 Continuous Glucose Monitoring System; NEUSPERA’s NUVELLA SYSTEM; Conformal Medical Initiates CONFORM Pivotal Trial; Meridian Launches New qPCR Master Mixes for Stool Samples; Sentinel Diagnostics Launches SENTiFIT 800
Conformal Medical Announces Launch of CONFORM Pivotal Trial On June 17, 2022, Conformal Medical Inc, is a medical device company manufacturing devices to avoid strokes in patients with non-valvular atrial fibrillation and developing next-generation LAAO technology. Its exclusive technology is intended to make le...
Read More...
May 26, 2022
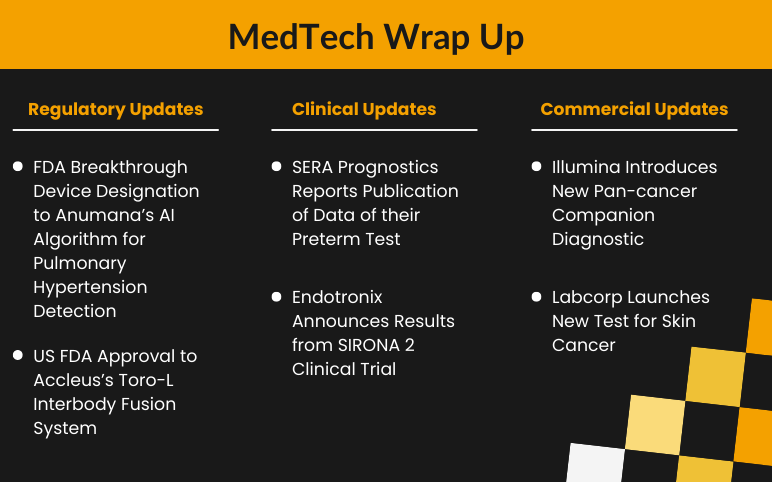
Accleus’s Toro-L Interbody Fusion System; Labcorp’s New Test for Skin Cancer; SERA Prognostics’s Preterm Test; Endotronix’s SIRONA 2 Clinical Trial; Anumana’s AI Algorithm to Detect ECG Pulmonary Hypertension; Illumina’s Pan-cancer Companion Diagnostic
Accleus Receives Regulatory Approval from US FDA for Toro-L Interbody Fusion System On May 19, 2022, the US Food and Drug Administration (FDA) granted the regulatory approval to Toro-L interbody fusion system developed by Accleus. The device is a biplanar expandable lateral implant designed for a minimal inserti...
Read More...
Apr 29, 2022
Emerging Market for Organ on a Chip
The organ on a chip is a microfluidic culture device that recapitulates the complicated structures and functions associated with the living human organs. The microdevices are composed of a clear flexible polymer that is almost the size of a USB memory stick and comprises hollow microfluidic channels lined up with l...
Read More...
Apr 07, 2022
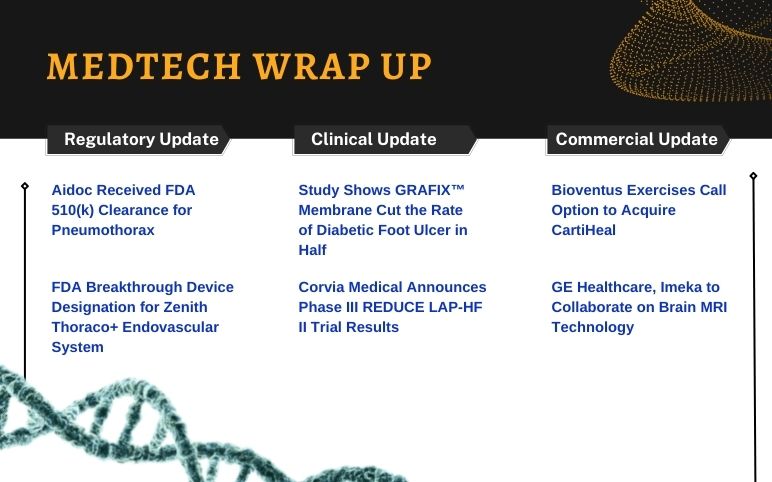
Aidoc’s Pneumothorax; Cook Medical’s Zenith Thoraco+ Endovascular System; GRAFIX Membrane Study; Corvia’s REDUCE LAP-HF II Trial Results; GE Healthcare and Imeka’s Collaboration; Bioventus to Acquire CartiHeal
Aidoc Expands AI Service to X-ray, Receiving FDA 510(k) Clearance for Pneumothorax On March 30, 2022, Aidoc, the leading developer of healthcare AI solutions, announced that its triage and notification of pneumothorax on X-ray exams has gained FDA 510(k) approval. Aidoc's other seven FDA-cleared clinical AI prod...
Read More...
Mar 24, 2022
Smithfield BioScience and BioCircuit’s Nerve Tape Device; Cynosure-Jeisys Medical’s Partnership; bioMérieux’s VITEK® MS PRIME new MALDI-TOF Mass Spectrometry Identification System; Artio Medical’s Gold Embolization Device; Heartpoint Global’s Implant System; ObvioHealth’s Virtual Urogynecology Clinical Trial
Smithfield BioScience and BioCircuit to Develop New Nerve Tape Device On March 16, 2022, BioCircuit Technologies, a National Institutes of Health (NIH)-funded medical device company primarily focused on developing and commercializing tissue repair and neural interfacing products, and Smithfield BioScience, a uni...
Read More...
Mar 17, 2022
NeuroLogica’s OmniTom Elite; Cordis’s S.M.A.R.T. RADIANZ Vascular Stent System; Rockley Photonics and Medtronic’s Collaboration; NovaSight’s Amblyopia Treatment Device; Cardiawave’s VALVOSOFT; OSF Ventures invests in wireless seizure detection sensor
NeuroLogica Announces FDA 510(k) Clearance for Photon Counting Computed Tomography Using OmniTom Elite On March 10, 2022, The state-of-the-art OmniTom Elite acquired 510(k) approval for the addition of Photon Counting Detector (PCD) technology, according to NeuroLogica Corp, a subsidiary of Samsung Electro...
Read More...
Mar 09, 2022
Evaluating the Top Players in the Global Bone Densitometers Market
Bone densitometers are the diagnostic systems with an enhanced X-ray technology known as dual-energy X-ray absorptiometry or DXA/DEXA technology that is capable of measuring the bone mineral density level. Bone mineral density (BMD) is an assessment of inorganic mineral content present in bone and is one of the ...
Read More...
Mar 03, 2022
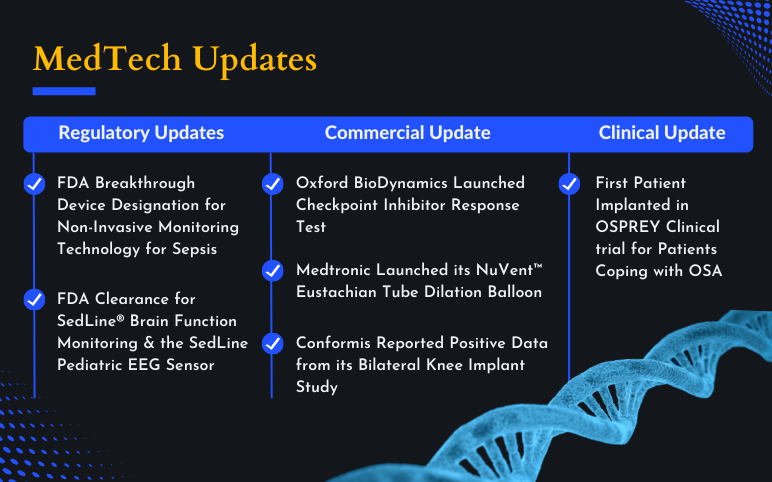
Noninvasix’s LIVOx™ Central Venous Oxygenation Monitor; LivaNova’s aura6000 System; Oxford BioDynamics’s EpiSwitch® CiRT; Masimo’s SedLine® Brain Function Monitoring and the SedLine Pediatric EEG Sensor; Medtronic’s NuVent™ Eustachian Tube Dilation Balloon; Conformis’s published data for Bilateral Knee Implant Study
Noninvasix Granted the US FDA Breakthrough Device Designation for Non-Invasive Monitoring Technology for Sepsis On February 23, 2022, Noninvasix, Inc. received the US FDA breakthrough device designation for its LIVOx™ Central Venous Oxygenation Monitor. It is a non-invasive device and provides real-time, ...
Read More...







-Agonist.png)

