MedTech News
Nov 11, 2021
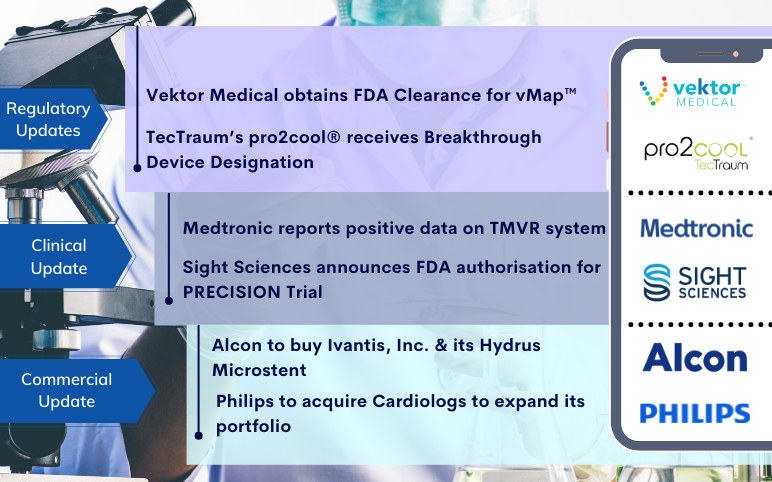
TecTraum’s pro2cool receives Breakthrough Device Designation; Vektor Obtains FDA Clearance for vMap; Medtronic’s TMVR system; Sight Sciences’s PRECISION Trial; Alcon to buy Ivantis; Philips to acquire Cardiologs
TecTraum’s pro2cool® receives FDA Breakthrough Device Designation for the Concussions treatment On November 03, 2021, TecTraum Inc., working to develop the world’s first point-of-care treatment for concussions, announced that the US Food and Drug Administration (FDA) has provided a Breakthrough Device to its f...
Read More...
Nov 04, 2021
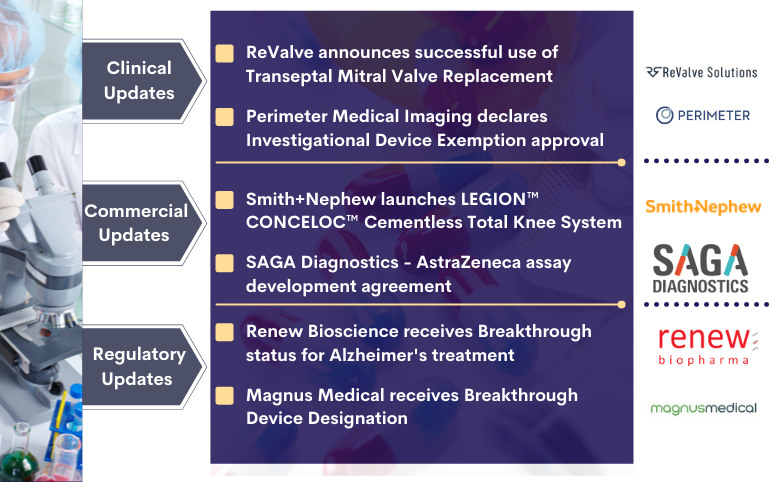
Smith+Nephew’s LEGION CONCELOC; Renew Bioscience’s Cerezen; Perimeter Medical’s ATLAS AI Project; SAGA Diagnostics-AZ’s Deal; Magnus Receives Breakthrough Device Designation; ReValve’s Transeptal Mitral Valve Replacement
ReValve Solutions, Inc. declares successful first human use of the Transeptal Mitral Valve Replacement On November 01, 2021, ReValve Solutions, Inc., a company operating in the field of transcatheter structural heart replacement and repair, announced the successful first human use of its transcatheter, Palmetto...
Read More...
Oct 28, 2021
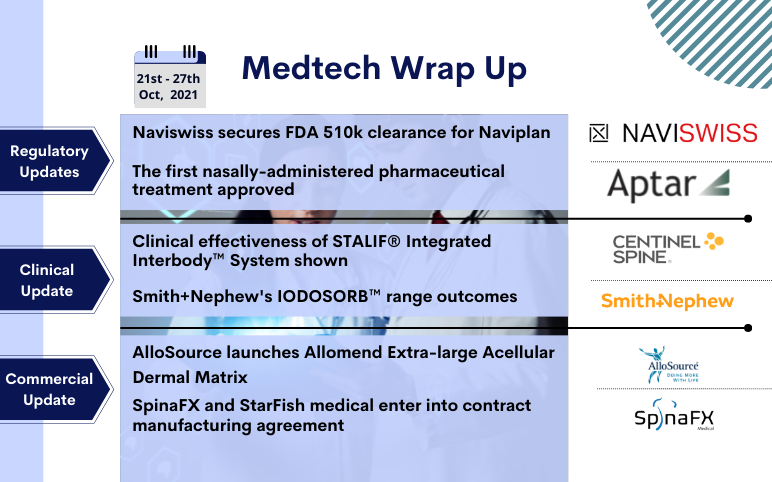
FDA 510k clearance to Naviswiss’s Naviplan; FDA approved to TYRVAYA; Centinel Spine’s STALIF Integrated Interbody System; Smith+Nephew’s IODOSORB; AlloSource’s AlloMend Extra-Large ADM; SpinaFX and StarFish’s strategic commercialisation contract
Naviswiss secures FDA 510k clearance for Naviplan, an advanced planning solution for hip replacement surgery On October 21, 2021, Naviswiss, a Swiss-based medical technology company, received approval from the FDA to commercialise Naviplan in the United States. Naviplan is a digital pre-operative planning ...
Read More...
Oct 21, 2021
Hologic to acquire Bolder Surgical; Breakthrough Device Designation to Biological Dynamics; Luminopia declares FDA approval; HistoSonics secures Breakthrough Device Designation; Phillips-Medisize expands worldwide; PolyNovo launches NovoSorb BTM tech
Hologic to acquire Bolder Surgical for USD160 Million, expanding its Surgical Franchise On October 14, 2021, Hologic, Inc., a global player in women's health, signed a definitive agreement to acquire Bolder Surgical. This US-based company offers advanced energy vessel sealing surgical devices for approxima...
Read More...
Oct 14, 2021
Bausch + Lomb’s LuxSmart Intraocular Lenses; Movair’s Luisa Life Supporting Ventilator; Cochlear’s Nucleus and Baha Systems; Laborie Acquires Pelvalon; Know Labs’s SARS-CoV-2 Research; Syntellix’s MAGNEZIX;
Syntellix obtains product approval for India On October 07, 2021, Syntellix obtained the license to bring five different product families of its revolutionary bioabsorbable magnesium-based implants to market from India's Central Drugs Standard Control Organisation (CDSCO) for the Indian market. With this approva...
Read More...
Oct 07, 2021
Amber Implants VCFix spinal system; iotaMotion’s iotaSOFT Insertion System; Boston Scientific acquires Baylis; ReCor’s Paradise Ultrasound Renal Denervation; Boston Scientific’s Ranger Drug-Coated Balloon; Natera’s Prospera
Amber Implants VCFix® spinal system obtains US FDA Breakthrough designation On October 05, 2021, the US Food and Drug Administration (FDA) granted the Breakthrough Device Designation to VCFix® spinal system, a next-generation spinal implant for spinal injuries developed by Amber Implants, a Netherlands-bas...
Read More...
Sep 30, 2021
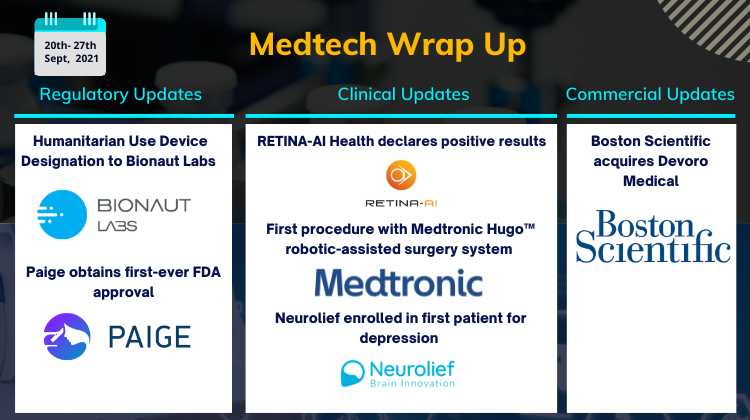
RETINA-AI Health declares positive results; Medtronic’s Hugo robotic-assisted surgery system; Boston Scientific acquires Devoro Medical; Paige obtains first-ever FDA approval
RETINA-AI Health, Inc. declares positive pivotal study results for the RETINA-AI Galaxy™ autonomous Diabetic Retinopathy screening device On September 20, 2021, RETINA-AI Health, Inc. announced optimistic results from the pivotal study of the RETINA-AI Galaxy™, a multi-device compatible autonomous ...
Read More...
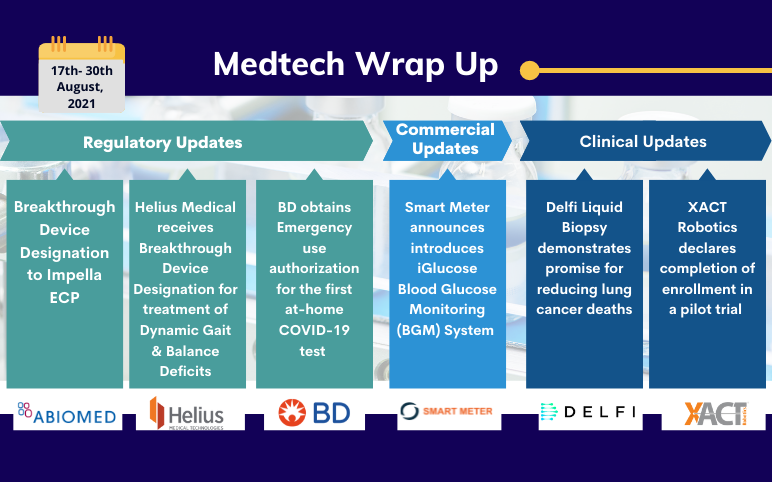
Sep 02, 2021
Abiomed’s Impella ECP; Helius’s PoNS Device; Smart Meter’s iGlucose BGM; XACT’s CT-Guided Percutaneous Procedures; BD’s Veritor At-Home COVID-19 Test; Delfi’s Liquid Biopsy
FDA awards Breakthrough Device Designation to Impella ECP On August 18, 2021, Abiomed received breakthrough device designation from the United States Food and Drug Administration (FDA) for its Impella ECP expandable percutaneous heart pump, the world’s smallest heart pump and the first to be compatible wit...
Read More...
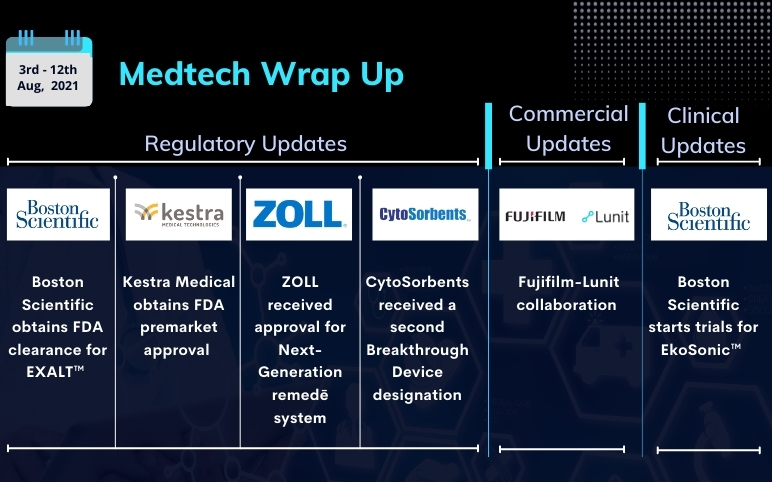
Aug 19, 2021
Boston Scientific’s EkoSonic; FDA clearance for EXALT; Kestra Medical’s ASSURE; ZOLL Next-Generation remedē system; CytoSorbents’ DrugSorb-ATR; Fujifilm-Lunit collaboration
Boston Scientific starts recruitment for randomized controlled trials for the EkoSonic™ Endovascular System On August 04, 2021, Boston Scientific Corporation started the enrolment in the HI-PEITHO clinical trial, which is a collaborative research study with the Pulmonary Embolism Response Team (PERT) Cons...
Read More...
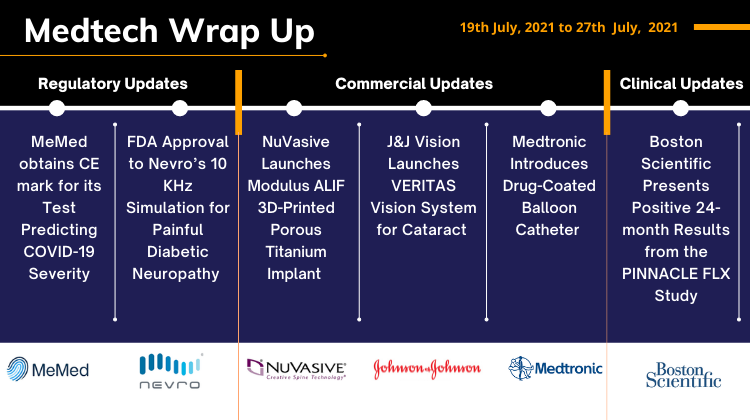
Aug 03, 2021
Nevro’s Spinal Cord Stimulation; Boston’s PINNACLE FLX; NuVasive’s Modulus ALIF; J&J’s VERITAS Vision System; Medtronic’s DCB Catheter; AngelMed’s Heart Attack Warning System
Nevro Received FDA Approval of its 10 kHz High-Frequency Spinal Cord Stimulation Therapy for Treatment of Chronic Pain Associated with Painful Diabetic Neuropathy On July 19, 2021, Nevro Corp. received approval for its Senza System, Nevro's unique 10 kHz stimulation, to treat chronic pain associated with Painful...
Read More...



-Agonist.png)

