Molecular Diagnostics
Nov 30, 2023
Baird Medical’s Microwave Ablation System; BrainSpec’s AI-Backed Solution for Non-Invasive Brain Chemistry Measurement; AWAK’s AI-Enabled Kidney Disease Prediction Tool; Revvity’s EONIS Q System; FormaPath Announced Advancement in Cancer Pathology; Pramand Launched CraniSeal Dural Sealant
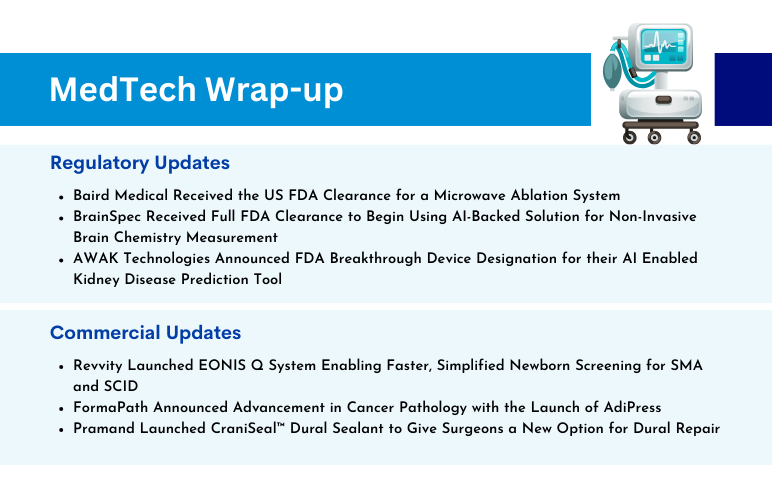
Baird Medical Received the US FDA Clearance for a Microwave Ablation System On November 22, 2023, Baird Medical announced the US Food and Drug Administration clearance of the company’s microwave ablation (MWA) system and disposable needles for use in the US. The clearance allows the use of the technology to a...
Read More...
Nov 09, 2023
SIBIONICS’s Ground-breaking GS1 Continuous Glucose Monitoring System; Abbott’s HPV Test to Run on Alinity m; CleanNA’s CE-IVD Molecular Diagnostics Product; Orthofix’s WaveForm L Interbody System; Alucent Biomedical’s AlucentNVS Technology; Surmodics’s TRANSCEND Trial Data
SIBIONICS Received CE Mark for Its Ground-breaking GS1 Continuous Glucose Monitoring System On November 01, 2023, SIBIONICS, the world's third-largest Continuous Glucose Monitoring System (CGM) brand, received the CE Mark for its revolutionary GS1 CGM. This significant milestone marks a momentous achiev...
Read More...
Nov 10, 2022
QuantuMDx & Menarini’s Agreement; TruClear System Launched by Medtronic in India; FDA 510(k) Clearance for TriVerse Primary Knee Replacement System; FDA Clearance to ProciseDx’s Reactive Protein (CRP) Assay and ProciseDx Instrument; Evolution Optiks’s Enrollment of LFR-260 Phoropter in US; Kardium First-in-Human Clinical Study of the New Globe Pulsed Field System
QuantuMDx and Menarini Announced an Agreement for the Distribution of the Q-POCTM Platform On November 2, 2022, QuantuMDx Group Limited, a UK-based developer of transformational Point-of-Need molecular diagnostics, and A.Menarini Diagnostics S.r.I. (Menarini), announced an exclusive distribution agreement for Qu...
Read More...
Mar 03, 2022
Noninvasix’s LIVOx™ Central Venous Oxygenation Monitor; LivaNova’s aura6000 System; Oxford BioDynamics’s EpiSwitch® CiRT; Masimo’s SedLine® Brain Function Monitoring and the SedLine Pediatric EEG Sensor; Medtronic’s NuVent™ Eustachian Tube Dilation Balloon; Conformis’s published data for Bilateral Knee Implant Study
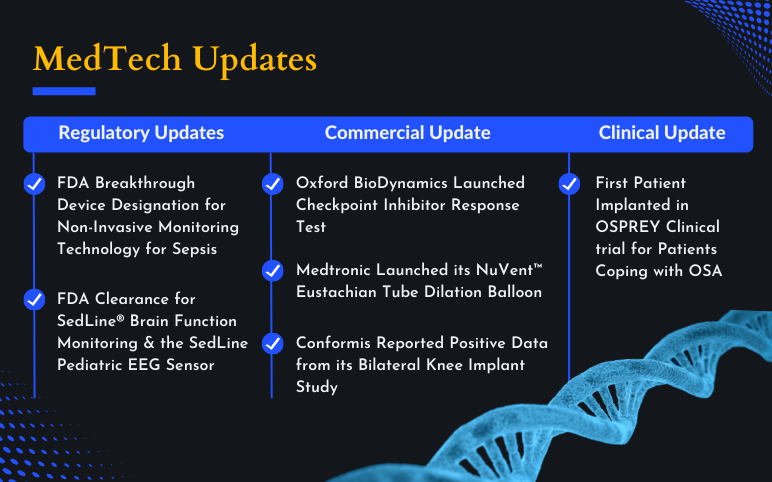
Noninvasix Granted the US FDA Breakthrough Device Designation for Non-Invasive Monitoring Technology for Sepsis On February 23, 2022, Noninvasix, Inc. received the US FDA breakthrough device designation for its LIVOx™ Central Venous Oxygenation Monitor. It is a non-invasive device and provides real-time, ...
Read More...


-Agonist.png)

