Multiple Myeloma Market
Dec 14, 2023
TALVEY’s Versatility Shines: Key Insights from MonumenTAL-2 Study Illuminate Effective Strategies in Relapsed/Refractory Multiple Myeloma
Date of Abstract presentation11th December 2023IndicationsMultiple MyelomaAbstract Number1014Abstract typeOral TALVEY (talquetamab) is a bispecific T-cell engaging antibody designed to target the CD3 receptor found on T-cells and G protein-coupled receptor class C group 5 member D (GPRC5D). The findings presente...
Read More...
Dec 13, 2023
DARZALEX FASPRO Quadruplet Regimen Demonstrates Striking Advancements in Treatment Outcomes for Newly Diagnosed Multiple Myeloma Eligible for Transplantation
Date of Abstract presentation12th December 2023IndicationsMultiple MyelomaAbstract NumberLBA1Abstract typeOral The PERSEUS study is being conducted in collaboration with the European Myeloma Network as a sponsor. PERSEUS is an ongoing, randomized, open-label, Phase III study comparing the efficacy and safety of ...
Read More...
Dec 13, 2023
Cilta-cel Demonstrates Prolonged and Deep Responses across Advanced Treatment Lines, Signifying Potential in Early Lenalidomide-Refractory Multiple Myeloma
Date of Abstract presentation11th December 2023IndicationsMultiple MyelomaAbstract Number1063Abstract typeOral According to the findings presented at the ASH 2023, the CARTITUDE-4 trial included a cohort of 419 patients. As of the clinical cut-off, 99 patients in the CARVYKTI arm and 66 in the standard of care (...
Read More...
Dec 13, 2023
Cilta-Cel’s Prolonged Impact: Deep Responses and Safety Insights from Earlier Lines of Therapy in Multiple Myeloma
Date of Abstract presentation11th December 2023IndicationsMultiple MyelomaAbstract Number1021Abstract typeOral CARVYKTI (ciltacabtagene autoleucel) is a B-cell maturation antigen (BCMA)-directed, genetically modified autologous T-cell immunotherapy, which involves reprogramming a patient’s T cells with a transge...
Read More...
Mar 05, 2021
Rare Cancer Market: A Global Crusade on what is a ‘less common cancer’?
Cancer is often challenging, however, diagnosis of rare cancer poses extra difficulties. Of all the diagnosed cancers, approximately 22% of the cases are rare cancers. According to the US National Cancer Institute, a cancer rare cancers as those with an incidence of fewer than 15 cases per 100 000 per year. Rare...
Read More...
Mar 29, 2019
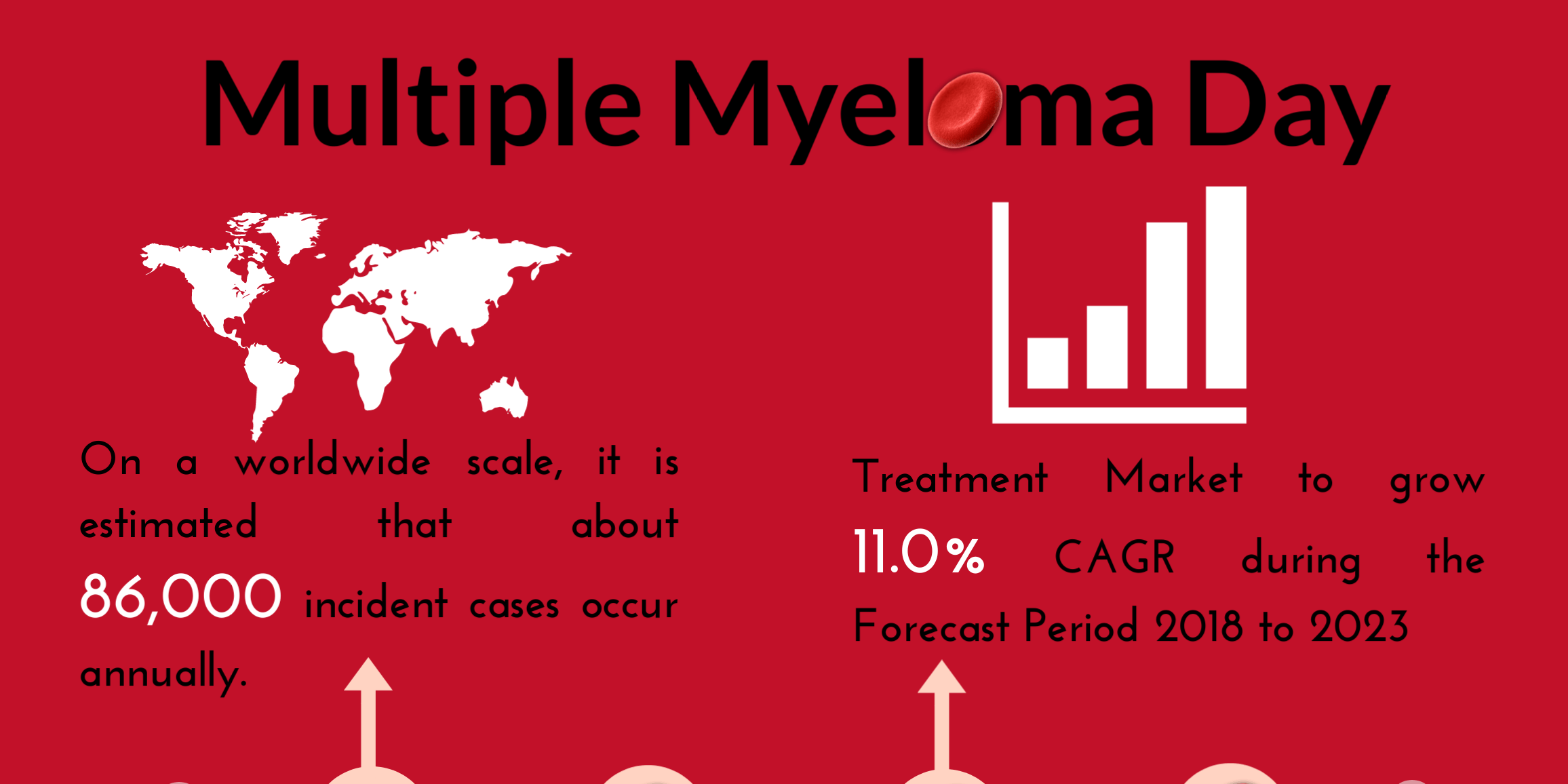
Multiple Myeloma Day
Multiple Myeloma Day Multiple Myeloma is relatively a rare disease. It accounts for approximately 1% of all cancers and 10% of all blood and bone marrow cancers. For more information on Multiple Myeloma.
Read More...

-Agonist.png)

