myasthenia gravis
Jan 28, 2025
FDA Approves LEQEMBI IV Dosing for Early Alzheimer’s; Vanda Accepts FDA Hearing on Tradipitant for Gastroparesis; Cartesian Gains FDA Protocol Approval for Myasthenia Gravis Trial; Zai Lab Secures FDA Orphan Status for DLL3 ADC in SCLC; Dyne Receives FDA Fast Track for DYNE-101 in Myotonic Dystrophy
FDA Approves LEQEMBI® (Lecanemab-Irmb) IV Maintenance Dosing for Early Alzheimer’s Disease Treatment Eisai Co., Ltd. and Biogen Inc. announced that the FDA has approved the Supplemental Biologics License Application (sBLA) for LEQEMBI (lecanemab-irmb), allowing intravenous (IV) maintenance dosing every four week...
Read More...
Jul 15, 2024
FcRn Inhibitors Being The Fastest Growing Class, Plans To Get Explored In 20+ Indications
Vyvgart enjoying strong commercial uptake whereas RYSTIGGO is preferred for MuSK+ Myasthenia gravis. What do the physicians believe? The neonatal Fc receptor (FcRn) inhibitor market is heating up, with a growing cast of leading contenders such as Argenx, JnJ, and UCB pharma, along with a few early-stage players ...
Read More...
Apr 09, 2024
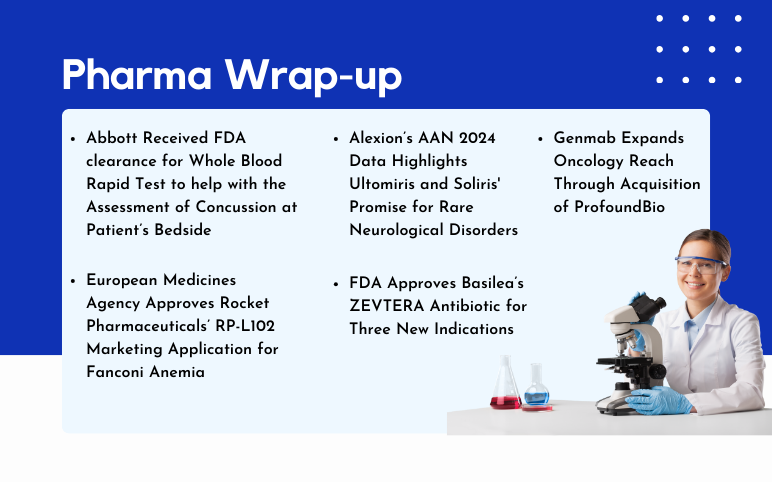
AstraZeneca and Daiichi Sankyo’s Enhertu US Approval; Basilea’s ZEVTERA FDA Approval; Genmab’s ProfoundBio Acquisition; Rocket Pharmaceuticals’ RP-L102 EMA Approval; Alexion’s Ultomiris and Soliris AAN 2024 Data
Enhertu Receives US Approval as First HER2-focused Treatment for Metastatic Solid Tumors, Independent of Tumor Origin AstraZeneca and Daiichi Sankyo's drug Enhertu (trastuzumab deruxtecan) has gained approval in the United States for treating adult patients with inoperable or metastatic HER2-positive (IHC 3+) so...
Read More...
Dec 19, 2023
Eisai Submits Marketing Authorization Application for Tasurgratinib; CHMP Issues Positive Opinion for Biogen’s SKYCLARYS; European Commission Approves Merck’s KEYTRUDA + Chemotherapy HER2-ve Gastric or GEJ Adenocarcinoma; BMS Provides Update on RELATIVITY-123 Trial; Kyverna Therapeutics Granted FDA Fast Track Designation for KYV-101; Verrica and Torii Pharma Announces Positive Top-line Results from a Confirmatory Phase 3 Trial of TO-208
Eisai Submits Marketing Authorization Application In Japan for Anticancer Agent Tasurgratinib For Biliary Tract Cancer With FGFR2 Gene Fusion Eisai Co., Ltd. has officially submitted a request for marketing authorization in Japan for tasurgratinib succinate, its internally developed tyrosine kinase inhibitor tar...
Read More...
Oct 24, 2023
FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved Moderate to Severe Plaque Psoriasis; FDA Approves BioMarin’s VOXZOGO; FDA Fast Track Designation to ANPD001 for Parkinson’s Disease; UCB Announces FDA Approval of ZILBRYSQ; EMA Granted Orphan Drug Designation to Lisata’s LSTA1
UCB announces FDA approval of ZILBRYSQ for the Treatment of Adults with Generalized Myasthenia Gravis On the 17th of October 2023, UCB (Euronext Brussels: UCB) made an announcement regarding the approval of ZILBRYSQ® (zilucoplan) by the US FDA for the management of generalized myasthenia gravis (gMG) in adult pa...
Read More...
Feb 07, 2023
Merck’s Keytruda Wins Another FDA Approval; Sanofi Pauses Trial of Myasthenia Gravis Drug, tolebrutinib; FDA Approves GlaxoSmithKline’s Jesduvroq; FDA IND Application Clearance for Hinova’s HP518; FDA Fast Track Designation to Endogena’s EA-2353; Amylyx Updates on Global Phase 3 PHOENIX Trial
Merck Wins Another FDA Approval for Blockbuster Keytruda Merck & Co arrived just two months after GSK celebrated a positive phase III result with its checkpoint inhibitor Jemperli as a first-line therapy for endometrial cancer. Keytruda (pembrolizumab) from Merck improved progression-free survival (PFS) vers...
Read More...
Apr 20, 2022
Top 12 Most Expensive Drugs in the US Healthcare Market
Drug pricing is one of the hottest topics in the healthcare segment. Several arguments have been put forward by people, governments, healthcare companies, and other organizations in favor of and against expensive medicines. Some of the drugs are so expensive that they are nearly out of reach of the common people. H...
Read More...
Mar 29, 2022
Sanofi’s Rare Disease Drug Xenpozyme’ Approval; FDA Approves Novartis’ Pluvicto; AstraZeneca’s Imfinzi Trial Result; FDA’s Green Signal to Argenx’ Vyvgart; ViiV Healthcare and J&J’s HIV Treatment Cabenuva; UCB’s FINTEPLA Approval; Cullinan’s Update for CLN-081; MEI Pharma & Kyowa’s Zandelisib Approval
Sanofi’s Rare Disease Drug Xenpozyme Wins World’s First Approval in Japan Sanofi’s Xenpozyme, also known as olipudase alfa, the world's first therapy for the rare disease Niemann-Pick type A/B and B, has been approved for the treatment of children and adults with non-CNS manifestations of Acid Sphingomyelinase D...
Read More...
Feb 08, 2022
Biogen’s Aduhelm; FDA Approves Sanofi’s Enjaymo; NHS & Orchard Signs a Deal; Bristol’s Breyanzi & Abecma; Pharming’s Leniolisib; Eli Lilly’s Verzenio Trial; UCB’ Zilucoplan Trials Result; Eli Lilly’s Alzheimer’s Drug Donanemab
Biogen's Aduhelm Marketing and Approval are Under Scrutiny in New FTC and SEC Investigations Biogen can't seem to get a break when it comes to the Alzheimer's disease drug Aduhelm. Along with limited sales and a constraining Medicare coverage plan, the medicine is now facing additional scrutiny as part of a pair...
Read More...
Jan 28, 2022
Evaluating the Upcoming Drugs in Pipeline for Major Autoimmune Diseases
The autoimmune diseases are frequently incapacitating and, in some circumstances, fatal. There are currently more than 80 identified autoimmune disorders, such as Rheumatoid Arthritis, Crohn's Disease, Plaque Psoriasis, Ulcerative Colitis, Myasthenia Gravis, and many more with symptoms ranging from mild to severe. ...
Read More...








-Agonist.png)

