Neurostimulation Devices
Mar 03, 2022
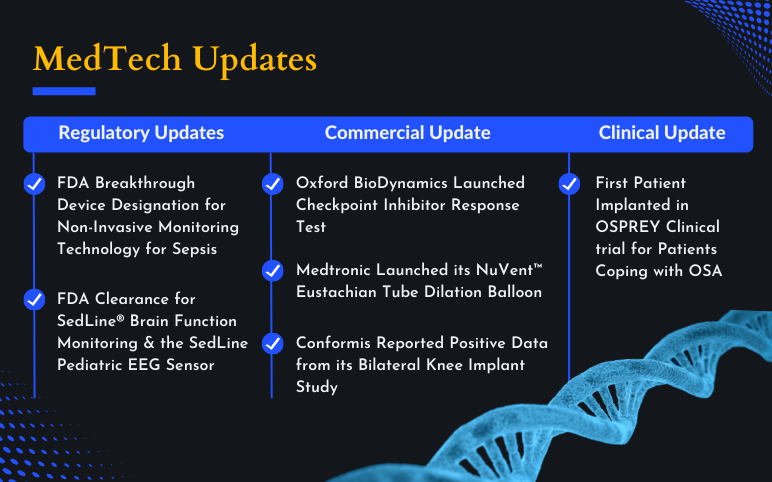
Noninvasix’s LIVOx™ Central Venous Oxygenation Monitor; LivaNova’s aura6000 System; Oxford BioDynamics’s EpiSwitch® CiRT; Masimo’s SedLine® Brain Function Monitoring and the SedLine Pediatric EEG Sensor; Medtronic’s NuVent™ Eustachian Tube Dilation Balloon; Conformis’s published data for Bilateral Knee Implant Study
Noninvasix Granted the US FDA Breakthrough Device Designation for Non-Invasive Monitoring Technology for Sepsis On February 23, 2022, Noninvasix, Inc. received the US FDA breakthrough device designation for its LIVOx™ Central Venous Oxygenation Monitor. It is a non-invasive device and provides real-time, ...
Read More...
-Agonist.png)

