news
Feb 16, 2023
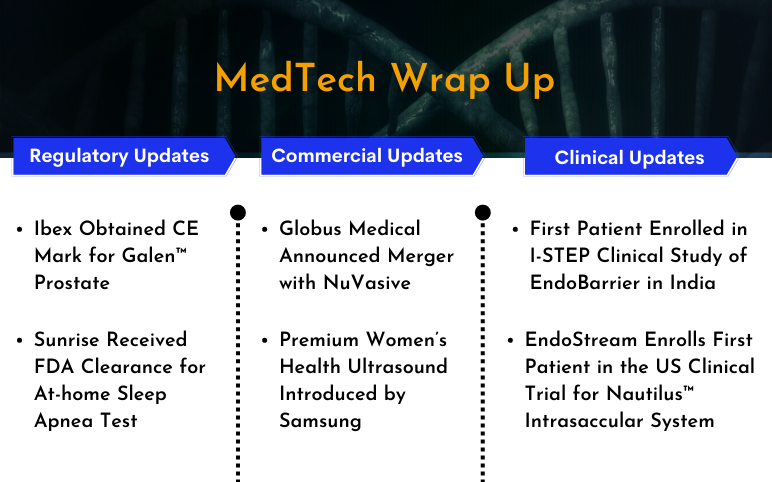
GI Dynamics’s I-STEP Clinical Study of EndoBarrier; EndoStream’s Clinical Trial for Nautilus™ Intrasaccular System; CE Mark for Ibex’s Galen™ Prostate; Sunrise’s At-home Sleep Apnea Test; Globus Medical Announced Merger with NuVasive; Boston Imaging’s HERA W10 Elite
First Patient Enrolled in I-STEP Clinical Study of EndoBarrier in India On February 13, 2023, GI Dynamics Inc., a medical device company, announced the enrollment of the first candidate in the I-STEP clinical trial in India. The trial aimed to assess the safety and effectiveness of the EndoBarrier® System for gl...
Read More...
Feb 09, 2023
LivaNova Launched SenTiva DUO; Candela Launched Matrix System; 3M Launched Medical Adhesive; MONARCH Platform Performed First Kidney Stones Removal Procedure; Biosense Webster Presented Data of HELIOSTAR Balloon Ablation Catheter; FDA 510(k) Clearance to Aspivix’s Cervical Stabilizer Carevix
LivaNova Launched SenTiva DUO, an Implantable Pulse Generator On February 3, 2023, LivaNova PLC, a market-leading medical technology and innovation company launched SenTiva DUO™, an implantable pulse generator (IPG) with a dual-pin header that delivers VNS Therapy™ for the treatment of drug-resista...
Read More...
Jan 31, 2023
Eli Lilly’s Jaypirca Approval; Novartis’ Adakveo EMA Review; Janssen’s CARTITUDE-4 Study of CARVYKTI; Negative Review on Ipsen’s Palovarotene; Gilead Sciences and Kite’s Yescarta NICE Recommendations; Daiichi Sankyo and AstraZeneca’s Enhertu EU Approval
FDA Approves Eli Lilly’s Jaypirca for Relapsed Mantle Cell Lymphoma Eli Lilly has received FDA approval for Jaypirca, a non-covalent BTK inhibitor, in relapsed mantle cell lymphoma (MCL) patients who have relapsed after treatment with other drugs in the class. Adult MCL patients who have previously received at l...
Read More...
Jan 24, 2023
BeiGene’s Brukinsa Approval; FDA Approval to Seagen’s TUKYSA; NICE Recommends Alnylam’s Amvuttra; FDA Approves Brenzavvy for Type 2 Diabetes; Roche’s Tecentriq to be Filed for Early-stage Liver Cancer; FDA Lifts Hold on Astellas’ Pompe Gene Therapy
FDA Approves BeiGene’s Brukinsa for CLL/SLL BeiGene's Brukinsa (zanubrutinib) for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has been approved by the US Food and Drug Administration. CLL is a common type of leukemia, accounting for approximately 25% of all new cases each year. SLL is...
Read More...
Jan 19, 2023
First Patient Enrolled in Kaizen Clinical Study in Japan; Koneksa’s At-Home Mobile Spirometry Clinical Trial; Medcura’s LifeGel™ Absorbable Surgical Hemostat; FDA Clearance to Abbott’s NAVITOR; Enovis’s DynaClip Delta™ and DynaClip Quattro™ bone staples; Bausch + Lomb Acquired AcuFocus
First Patient Enrolled in Kaizen Clinical Study in Japan On January 11, 2023, Cardiovascular Systems, Inc., a medical device company developing and commercializing innovative interventional treatment systems for patients suffering from peripheral and coronary artery disease, announced the initiation of the...
Read More...
Jan 10, 2023
Ipsen to Acquire Albireo; Chiesi Farmaceutici to Buy Amryt Pharma; Takeda Presents Phase III Results of TAK-755 for cTTP; ACELYRIN Acquires ValenzaBio; FDA Approves LEQEMBI for Alzheimer’s Disease; Orphan Drug Designation to Lantern Pharma’s LP-284 for MLL
Ipsen to Acquire Albireo for USD 952 Million Ipsen, a French pharmaceutical company, has offered USD 42 per share for the US biotech Albireo and its rare disease treatment Bylvay, continuing a recent spree of merger and acquisition activity. Albireo, a Boston-based business, is valued at USD 952 million under th...
Read More...
Jan 03, 2023
Gilead Buys Out Rights to Cancer Therapy from Jounce; FDA Places Clinical Hold on Biogen’s Orelabrutinib; Pfizer Announces Phase 3 BENEGENE-2 Study Result; FDA Approves MediWound’s NexoBrid; UCB Announces FDA Acceptance of BLA Resubmission for Bimekizumab; FDA Approves TG Therapeutics’ Briumvi
Gilead Buys Out Rights to Cancer Therapy from Jounce for USD 67 Million Gilead Sciences must have liked what it saw in a two-year-old collaboration with Jounce Therapeutics for CCR8-targeting cancer immunotherapy because the company has just agreed to own the program fully. The drug in question, GS-1811 (formerl...
Read More...
Dec 27, 2022
Gilead Sciences’ Sunlenca Approval; FDA Approves Roche’s CD20xCD3 Bispecific Antibody Lunsumio; EU Approves AstraZeneca’s Imfinzi Plus Chemo; Pfizer Files Blockbuster Hope Etrasimod for Ulcerative Colitis; FDA Approves Mosunetuzumab for R/F Follicular Lymphoma; FDA Breakthrough Therapy Designation to Adagrasib Plus Cetuximab for KRAS G12C–Mutated Advanced CRC
FDA Approves Gilead Sciences’ Sunlenca Sunlenca, a Gilead Sciences therapy for people with multidrug-resistant (MDR) HIV infection that only needs to be taken twice a year, has received FDA approval for the second time of asking. Sunlenca, which is based on the HIV capsid inhibitor lenacapavir, is intended to be...
Read More...
Dec 22, 2022
Medtronic’s Hugo Robotic-Assisted Surgery System Trial; Insightec’s Pivotal LIBERATE Clinical Trial; FDA Granted EMA for Thermo Fisher Scientific’s Monkeypox Test; Life Spine’s Trulift Lateral Expandable Spacer System & Lateral Pate System; Fujifilm & Inspirata Announces Asset Purchase Agreement; Abbott Launched SCS System for Chronic Pain
Medtronic Announced First Patient Enrolment for Hugo™ Robotic-Assisted Surgery System in US Clinical Trial On December 15, 2022, Medtronic, a global healthcare technology leader, announced that the first patient was enrolled in the Expand URO US clinical trial for the Hugo™ robotic-assisted surgery...
Read More...
Dec 20, 2022
Fourth FDA Approval for AbbVie’s Vraylar; FDA Approves Ferring’s Adstiladrin for NMIBC; Merck and Moderna’s mRNA Cancer Vaccine Trial; EMA Recommends the CSL’s Gene Therapy for Hemophilia B; CHMP Backs Amicus’ Pompe Disease Therapy; Takeda Announces the Phase 3 AURORA Study Result
AbbVie Secures Fourth FDA Approval for Vraylar AbbVie has received its fourth FDA approval for Vraylar, adding major depressive disorder (MDD) adjunctive therapy to a list that includes schizophrenia and manic and depressive episodes in bipolar disorder. According to AbbVie, the approval makes Vraylar (cariprazi...
Read More...








-Agonist.png)

