news
Sep 20, 2022
AstraZeneca’s Danicopan Trial; CHMP Recommends Sanofi/AstraZeneca’s nirsevimab; Akero’s NASH Drug Trial; FDA Grants Orphan Drug Status to SY-5609; BMS’s Opdivo Trial Results; Pfizer to File for FDA Approval for Meningitis Vaccine; EMA Orphan Drug Designation to CAN-2409; FDA Starts Priority Review of Chiesi ‘s velmanase alfa
AstraZeneca’s Danicopan Shows Positive Results in Phase III Trial Danicopan, an oral Factor D inhibitor developed by AstraZeneca, was expected to fail a phase II trial in rare kidney disease in 2020, but a new readout could revive the drug. Danicopan (ALXN2040) has demonstrated efficacy as an adjunct treatment f...
Read More...
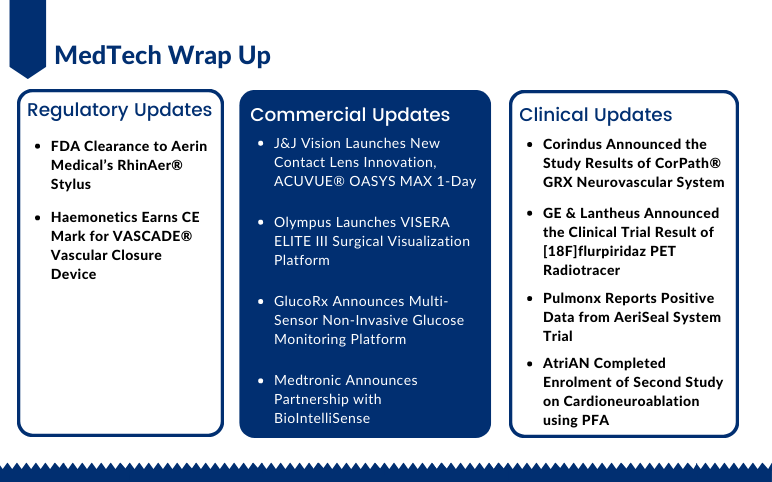
Sep 15, 2022
Aerin Medical’s RhinAer Stylus; CE Mark for Haemonetics’s VASCADE Vascular Closure Device; J&J Vision Launches ACUVUE OASYS MAX 1-Day; Olympus Launches VISERA ELITE III Surgical Visualization Platform; GlucoRx’s Non-Invasive Glucose Monitoring Platform; Medtronic-BioIntelliSense’s Partnership; Corindus’s CorPath GRX Neurovascular System Trial; GE & Lantheus [18F]flurpiridaz PET Radiotracer Trial; Pulmonx’s AeriSeal System Trial; AtriAN’s Second Study on Cardioneuroablation
Johnson & Johnson Vision Launches New Contact Lens Innovation ACUVUE® OASYS MAX 1-Day for Meeting the Needs of Digitally Intense Lifestyles On September 12, 2022, Johnson & Johnson Vision, a part of Johnson & Johnson and a global leader in the eyecare market, had announced the launch of its newest in...
Read More...
Sep 13, 2022
Amgen Announced the Result of its CodeBreak-200 Trial; FDA Clears Bristol-Myers Squibb’s Deucravacitinib; BioNTech’s Amplified CAR-T Therapy; TIL Therapy Improves on Yervoy in Melanoma; GSK’s Daprodustat will have to face FDA Advisory Committee; Breakthrough Therapy Status to Pfizer’s Group B Strep Vaccine; EU Approves Gilead’ Tecartus; Gilead’ Trodelvy Results in TROPiCs-02 Trial
Amgen Reveals the Top-line Result of its CodeBreak-200 trial of Lumakras in Lung Cancer The top-line result of Amgen's CodeBreak-200 trial of Lumakras in lung cancer was presented in abstract form at ESMO two weeks ago, showing a 34% improvement in progression-free survival (PFS) compared to chemotherapy. The fu...
Read More...
Sep 08, 2022
eCential Robotics’s Surgical Robotic Platform for Spine Surgery; Baxter Gets 510(k) Clearance for Syringe Pump; Medical Microinstruments’s NanoWrist Instruments; Magnus’ SAINT Neuromodulation System; Henry Schein Acquires Midway Dental Supply
eCential Robotics Receives FDA Clearance for its Surgical Robotic Platform for Spine Surgery The FDA has approved a robotic spinal surgery platform designed to assist human surgeons by automating several steps of spinal procedures. The platform, developed by eCential Robotics, combines intraoperative 2D and 3D i...
Read More...
Sep 06, 2022
AstraZeneca’s Imfinzi for Biliary Tract Cancer; FDA Clears Boehringer’s Spesolimab; Novo Nordisk to Acquire Forma Therapeutics; Sanofi’s Xenpozyme Approved for ASMD; Another FDA Approval to Azurity’s Konvomep; Amgen’s Lumakras Trial Results; FDA Grants Priority Review to Sanofi & Sobi’s efanesoctocog alfa; Neurocrine Bio to Takeover UK Biotech Diurnal
FDA Approves AstraZeneca’s Imfinzi for Biliary Tract Cancer Imfinzi, a checkpoint inhibitor developed by AstraZeneca, has been approved by the FDA as the first immunotherapy for biliary tract cancer (BTC), a rare and aggressive form of cancer with few treatment options. Imfinzi (durvalumab) has been approved by ...
Read More...
Sep 01, 2022
Miracor Medical’s Picso Pivotal Study; Medtronic’s Extravascular ICD; Galaxy Medical’s CENTAURI Pulsed Electric Field System; UltraSight’s Novel Cardiac AI Technology; Oticon Launched the World’s Smallest Hearing Aid; Medtronic Completes Acquisition of Affera
FDA Approved IDE for Picso® Pivotal Study of Miracor Medical On August 23, 2022, Miracor Medical SA, a provider of innovative solutions for the treatment of severe cardiac diseases, announced that the FDA has approved an Investigational Device Exemption (IDE), allowing the company to begin a pivotal study with i...
Read More...
Aug 30, 2022
Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to Spin Off Sandoz Business; Alcon to Buy Aerie Pharma; Fast Track Designation to Merck’s MK-2060; FDA Approves Ibrutinib for Chronic GvHD; French Authorities Clears BrainVectis’s Clinical Trial; Takeda’s Dengue Vaccine TAK-003 Gets Approval in Indonesia
Aktis Oncology Raises USD 84 Million To Advance Novel Targeted Alpha Radiopharmaceuticals Aktis Oncology has raised an additional USD 84 million in its Series A round, adding to the USD 72 million raised last year to help bring its radiopharmaceuticals to market. The extension to the first round included Merck's...
Read More...
Aug 25, 2022
Edwards’s Pascal Precision System; Abbott’s New Spinal Cord Stimulation Device; Imagin to Acquire enCAGE Coil Precision Ablation System; Boston Heart Diagnostics Launches LipidSeqTM; Rocket VR Health Partners with Penn Medicine’s Abramson Cancer Center; Thermedical’s Clinical Trial to Treat Ventricular Tachycardia
Edwards Pascal Precision Transcatheter Mitral And Tricuspid Valve Repair System Receives CE Mark On August 17, 2022, Edwards Lifesciences Corporation, an American medical technology company headquartered in Irvine, California, specializes in artificial heart valves and hemodynamic monitoring. The company announc...
Read More...
Aug 23, 2022
EMA to review Cidara Therapeutics’ Rezafungin; EU Approves Gilead Sciences’ Sunlenca; FDA Approves Axsome’s Auvelity; EU Marketing Authorisation to Oncopeptides’s Pepaxti; Merck Signs $ 3.5 B Deal with Orna; FDA to review AstraZeneca and Merck’s Lynparza; FDA Approval to Bluebird’s Zynteglo; FDA Decision Date for GSK’s Momelotinib
EU Regulator Starts its Review of Cidara Therapeutics’ Candidiasis Therapy Rezafungin The EU regulator has begun its review of Cidara Therapeutics’ once-weekly antifungal rezafungin, with a decision expected next year as a new option for serious, invasive candida infections. The application is based on the ReSTO...
Read More...
Aug 18, 2022
CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath Secures $10 Million Funding; BD, Accelerate Diagnostics Announce Collaboration; Avails Medical’s Clinical Trials for eQUANT; Movano Ring Exceeds Accuracy Targets for SpO2 & Heart Rate Monitoring
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System On August 09, 2022, CereVasc, Inc., a privately held, clinical-stage medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved ...
Read More...








-Agonist.png)

