news
Mar 24, 2022
Smithfield BioScience and BioCircuit’s Nerve Tape Device; Cynosure-Jeisys Medical’s Partnership; bioMérieux’s VITEK® MS PRIME new MALDI-TOF Mass Spectrometry Identification System; Artio Medical’s Gold Embolization Device; Heartpoint Global’s Implant System; ObvioHealth’s Virtual Urogynecology Clinical Trial
Smithfield BioScience and BioCircuit to Develop New Nerve Tape Device On March 16, 2022, BioCircuit Technologies, a National Institutes of Health (NIH)-funded medical device company primarily focused on developing and commercializing tissue repair and neural interfacing products, and Smithfield BioScience, a uni...
Read More...
Mar 22, 2022
Bristol Myers’ Opdivo combo Opdualag for Melanoma; Biogen’s Aduhelm; Marinus’ Ztalmy for CDKL-5 Deficiency Disorder; Merck’s Keytruda + Lynparza; Vitaris’s Breyna; Moderna’s Second COVID-19 Booster Shot; Takeda’s Exkivity; BMS’s First LAG-3 Checkpoint Inhibitor
NHS Grants Fast Track Access to Takeda’s Exkivity Takeda has secured UK approval for its lung cancer therapy Exkivity, with an NHS access deal that could see it prescribed to patients within the next few weeks. The Medicines and Healthcare products Regulatory Agency has granted conditional marketing authorisa...
Read More...
Mar 17, 2022
NeuroLogica’s OmniTom Elite; Cordis’s S.M.A.R.T. RADIANZ Vascular Stent System; Rockley Photonics and Medtronic’s Collaboration; NovaSight’s Amblyopia Treatment Device; Cardiawave’s VALVOSOFT; OSF Ventures invests in wireless seizure detection sensor
NeuroLogica Announces FDA 510(k) Clearance for Photon Counting Computed Tomography Using OmniTom Elite On March 10, 2022, The state-of-the-art OmniTom Elite acquired 510(k) approval for the addition of Photon Counting Detector (PCD) technology, according to NeuroLogica Corp, a subsidiary of Samsung Electro...
Read More...
Mar 15, 2022
AstraZeneca and Merck’s Lynparza; Venatorx’s cefepime-taniborbactam; AbbVie’s Qulipta; AstraZeneca’s IL-5 inhibitor Fasenra; Sandoz Acquires Coalesce; Incyte’s Opxelura; Bayer’s Nubeqa; NICE Rejects Keytruda Plus Chemotherapy
FDA Clears AstraZeneca and Merck’s Lynparza as an Adjuvant Breast Cancer Therapy The FDA approved the AstraZeneca and Merck drug for a specific group of patients with high-risk early breast cancer after chemotherapy treatment, either before or after surgery. Lynparza cements its position as the best-selling PARP...
Read More...
Mar 10, 2022
Bioventus’s StimRouter Pain Management Device; FDA Approval for eCoin Therapy; SurGenTec Launches ION Screw; Exactech’s Humeral Reconstruction Prosthesis; Boston Scientific’s WATCHMAN FLX LAAC Device; PathMaker Neuro’s MyoRegulator
Bioventus Receives 501(k) Clearance for StimRouter Pain Management Device On March 1, 2022, the US Food and Drug Administration (FDA) gave Bioventus' StimRouter Neuromodulation System 510(k) approval. The next-generation pain treatment device is intended to treat chronic pain caused by peripheral nerves, ex...
Read More...
Mar 08, 2022
Lilly & Boehringer’s Jardiance; BMS Secures a Neoadjuvant Market for Opdivo; Amgen & NHS Signs a Deal; Roche’s Alzheimer Drug gantenerumab; Pfizer’s RSV Vaccine; Civica’s Insulin Biosimilars; Eli Lilly & Incyte’s JAK inhibitor Olumiant; Natco Pharma Launches Revlimid Generic
Eli Lilly, Boehringer Ingelheim’s Jardiance Giving Competition to AstraZeneca’s Farxiga with EU Approval Jardiance, the diabetes blockbuster from Eli Lilly and Boehringer Ingelheim, recently received a Heart Failure label expansion in the United States. Now, the drug is expanding into Europe, with a nod that mig...
Read More...
Mar 03, 2022
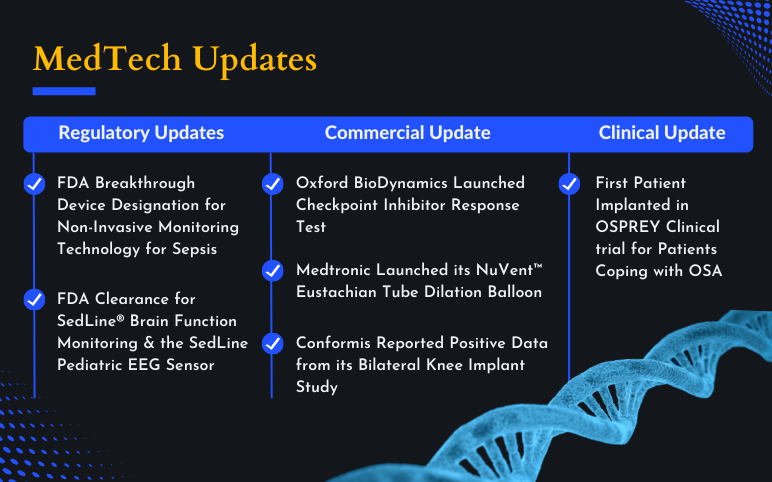
Noninvasix’s LIVOx™ Central Venous Oxygenation Monitor; LivaNova’s aura6000 System; Oxford BioDynamics’s EpiSwitch® CiRT; Masimo’s SedLine® Brain Function Monitoring and the SedLine Pediatric EEG Sensor; Medtronic’s NuVent™ Eustachian Tube Dilation Balloon; Conformis’s published data for Bilateral Knee Implant Study
Noninvasix Granted the US FDA Breakthrough Device Designation for Non-Invasive Monitoring Technology for Sepsis On February 23, 2022, Noninvasix, Inc. received the US FDA breakthrough device designation for its LIVOx™ Central Venous Oxygenation Monitor. It is a non-invasive device and provides real-time, ...
Read More...
Mar 01, 2022
GSK’s Covifenz; Idorsia’s Quviviq; GSK’s ZEJULA; EMA Expands its Nod for BMS, Eli Lilly, and Novartis Drugs; Cantex Secures Global Licence to Develop Azeliragon; Biocon Acquires Viatris’ Biosimilar Business
GSK’s Covifenz, A Plant-Based COVID Vaccine, Receives First Approval The recombinant COVID-19 vaccine developed by Medicago, now known as Covifenz, has received approval in Canada, the company's home country. Covifenz employs Coronavirus-Like Particle (CoVLP) technology, with the vaccine consisting of recombinan...
Read More...
Feb 24, 2022
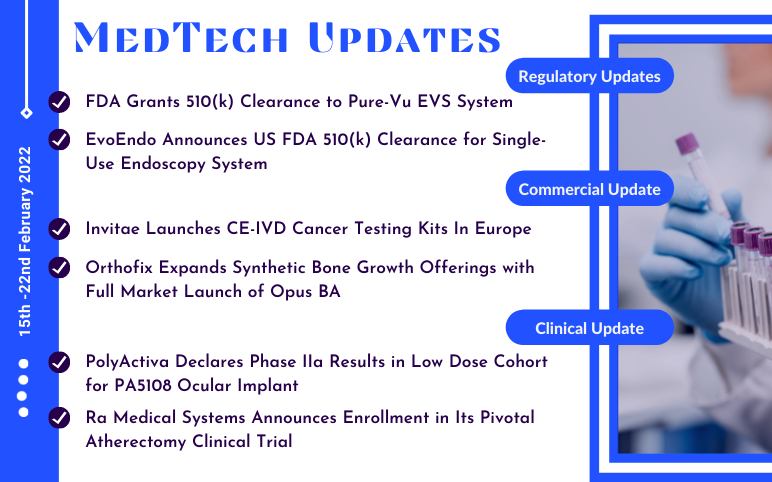
Motus GI’s Pure-Vu EVS System; EvoEndo’s Single-Use Unsedated TNE System; Orthofix’s OpusTM BA; Invitae’s CE-IVD Cancer Testing Kits; PolyActiva’s PA5108 Ocular Implant; Ra Medical’s DABRA excimer laser system
US FDA grants 510(k) clearance to Motus GI’s Pure-Vu EVS System On February 15, 2022, The Pure-Vu® EVS System received 510(k) clearance from the US Food and Drug Administration, according to Motus GI Holdings, Inc., a medical technology company that provides endoscopy solutions that improve clinical outcomes and...
Read More...
Feb 22, 2022
Sandoz’s Generic Revlimid; Agios’ Pyrukynd; Organon Announces 4Q & Full-year Earnings Report; BMS’ CAR-T Drug Breyanzi; Sanofi & Regeneron’s Dupixent Trial; AZ & Daiichi’s Drug Enhertu; Bayer’s Drug Kerendia; Lilly Releases Mirikizumab Data
Sandoz Launches generic Revlimid in 19 European Countries, Bringing a Flood of Competition to BMS' Megablockbuster Since Bristol Myers Squibb acquired Celgene and its megablockbuster Revlimid, the company has been bracing for the day when the multiple myeloma superstar would face generic competition. Sandoz, a s...
Read More...








-Agonist.png)

