news
Feb 22, 2022
Sandoz’s Generic Revlimid; Agios’ Pyrukynd; Organon Announces 4Q & Full-year Earnings Report; BMS’ CAR-T Drug Breyanzi; Sanofi & Regeneron’s Dupixent Trial; AZ & Daiichi’s Drug Enhertu; Bayer’s Drug Kerendia; Lilly Releases Mirikizumab Data
Sandoz Launches generic Revlimid in 19 European Countries, Bringing a Flood of Competition to BMS' Megablockbuster Since Bristol Myers Squibb acquired Celgene and its megablockbuster Revlimid, the company has been bracing for the day when the multiple myeloma superstar would face generic competition. Sandoz, a s...
Read More...
Feb 17, 2022
Össur Launches POWER KNEE; DePuy Synthes Acquires CrossRoads Extremity Systems; Myomo’s MyoPro; Accuray’s TomoTherapy System; Theradaptive’s Spinal Fusion Implant; Senseonics’s Eversense E3 CGM System
Össur Launches World’s Actively Powered Lower Limb Bionic Prosthesis- New Power Knee for Amputees On February 09, 2022, Össur announced the launch of the new POWER KNEE, the first actively powered microprocessor-based prosthetic knee in the world. This device is aimed at treating patients with above-knee amputat...
Read More...
Feb 10, 2022
Stryker’s Tornier Shoulder Arthroplasty; Genesis Acquires JC Medical; FDA Clearance to Single-Use Gastroscope; BioCardia’s CardiAMP Cell Therapy System; Abbott’s Dual-Chamber Leadless Pacemaker; atHeart’s ASCENT ASD U.S. IDE
BioCardia Receives FDA Breakthrough Device Designation for CardiAMP Cell Therapy System for Heart Failure On February 03, 2022, The US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the CardiAMP® Cell Therapy System for the treatment of heart failure, according to BioCardia®, ...
Read More...
Feb 08, 2022
Biogen’s Aduhelm; FDA Approves Sanofi’s Enjaymo; NHS & Orchard Signs a Deal; Bristol’s Breyanzi & Abecma; Pharming’s Leniolisib; Eli Lilly’s Verzenio Trial; UCB’ Zilucoplan Trials Result; Eli Lilly’s Alzheimer’s Drug Donanemab
Biogen's Aduhelm Marketing and Approval are Under Scrutiny in New FTC and SEC Investigations Biogen can't seem to get a break when it comes to the Alzheimer's disease drug Aduhelm. Along with limited sales and a constraining Medicare coverage plan, the medicine is now facing additional scrutiny as part of a pair...
Read More...
Feb 03, 2022
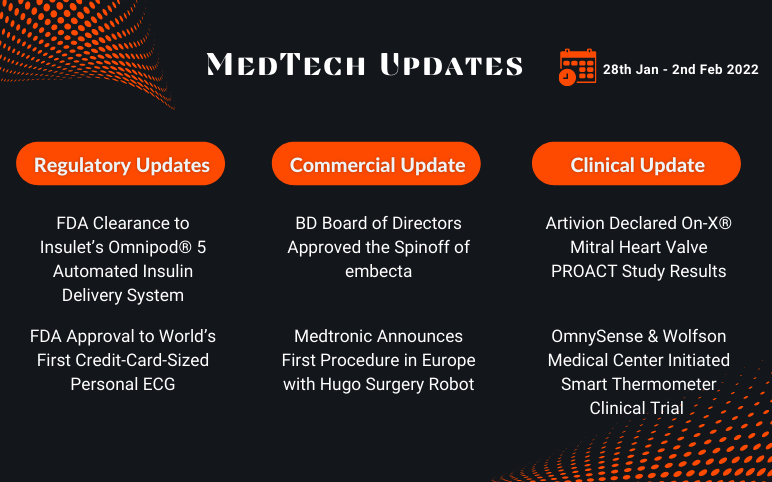
Insulet’s Omnipod 5 Automated Insulin Delivery System; Alivecor’s KardiaMobile Card; BD Board Approved the Spinoff of embecta; Medtronic’s Hugo Surgery Robot; Artivion’s On-X® Mitral Heart Valve PROACT; OmnySense’s Smart Thermometer Clinical Trial
Insulet Received FDA Clearance for its Omnipod® 5 Automated Insulin Delivery System, First Tubeless System with Smartphone Control On January 28, 2022, Insulet Corporation, a market leader in developing tubeless insulin pumps with its product, Omnipod®, received the FDA approval for its Omnipod® 5 Automated Insu...
Read More...
Feb 01, 2022
Immunocore’s Kimmtrak; Samsung Acquires Biogen’s Biosimilar Unit; Novavax’s COVID-19 Vaccine; CHMP Approves Dupixent (dupilumab); Glenmark’s High Blood Pressure Treatment; Roche’s Faricimab; GBT’s Voxelotor
Immunocore Receives FDA Approval for the First Uveal Melanoma Treatment, As Well As the First T-cell Receptor Therapeutics Uveal Melanoma, an aggressive eye cancer, has proven to be a tough nut to crack for researchers seeking a cure. However, with the approval of a new treatment, those with the disorder will ha...
Read More...
Jan 27, 2022
Fluidx Medical’s GPX Embolic Device; Cardiovascular Systems & OrbusNeich’s Scoreflex Scoring Balloon; BENDIT’s BENDIT21 Neuro Catheter; Cook Medical Drug-eluting Stent; Zimmer Biomet’s Spinoff; Smith+Nephew’s PICO 7 and PICO 14 System
GPX Embolic Device Success in Challenging Tumor Case On January 20, 2022, In a multi-center clinical study, Fluidx Medical's GPX Embolic Device was utilized to efficiently devascularize a massive tumor with several feeding vessels. The GPX Embolic Device is a cutting-edge embolic device that allows for easy prep...
Read More...
Jan 25, 2022
Merck’s Gefapixant; Pfizer’s Somatrogon; Gilead’s Viklury; AbbVie’s Skyrizi; Gilead’s Zydelig Approvals; Lantern’s LP-184; Polpharma to Acquire Advent
Merck’s Chronic Cough Med Gefapixant Receives A Red Flag From the FDA, Requests For More Data On 24th January 2022, Merck & Co. announced FDA rejection for its New Drug Application (NDA) of Gefapixant (experimental drug) for unexplained or chronic cough. FDA asked for additional information on the drug, rela...
Read More...
Jan 20, 2022
Suneva Medical-Viveon Health merger update; Smith+Nephew acquires Engage Surgical; Abiomed’s preCARDIA technology; Medtronic’s DTM Spinal Cord Stimulation; Endo Tools Therapeutics’s Endomina System; Diadem’s AlzoSure predict prognostic blood test
Suneva Medical Inc. inked a merger agreement with Viveon Health On January 12, 2022, Suneva Medical Inc., a leading regenerative aesthetics company, and Viveon Health Acquisition Corp., a particular purpose acquisition company, entered into a definitive merger agreement to establish a new regenerative aesthetics...
Read More...
Jan 18, 2022
Algernon’s NP-120 (ifenprodil); Pieris’s cinrebafusp alfa (PRS-343) clinical trial; Gaumard Scientific’s multidisciplinary patient simulator HAL S5301; Hekka Labs’s decentralized healthcare ecosystem
Algernon receives positive FDA feedback on Phase IIb chronic cough trial The US Food and Drug Administration (FDA) has given positive feedback for Algernon Pharmaceuticals’ Phase IIb clinical trial of NP-120 (ifenprodil) for chronic cough treatment. A receptor antagonist of N-methyl-D-aspartate (NMDA), ifenp...
Read More...









-Agonist.png)

