news
Dec 02, 2021
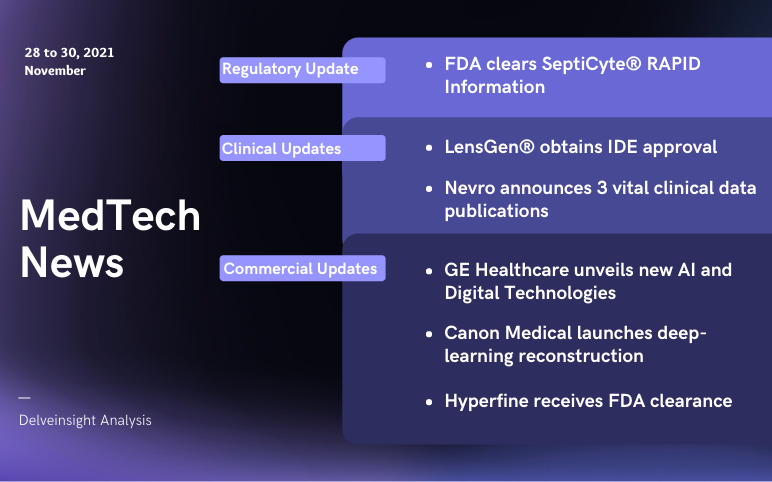
LensGen’s Juvene Intraocular Lens; Nevro announces clinical data publications; FDA Clearance to Immunexpress’s SeptiCyte; GE unveils AI and Digital Technologies; Canon launches PIQE DLR and SilverBeam; Hyperfine’s Swoop gets FDA clearance
Canon Medical launches super-resolution deep-learning reconstruction for Cardiac CT scans On November 28, 2021, Canon Medical launched the Precise IQ Engine (PIQE) DLR and SilverBeam filter in the recent version of the Aquilion ONE / PRISM edition. PIQE, super-resolution deep-learning reconstruction techno...
Read More...
Nov 30, 2021
GE Healthcare-Optellum’s collaboration; Ocugen’s Covid-19 vaccine trial; Blueprint to acquire Lengo Therapeutics; Therapixel’s Mammoscreen
GE Healthcare, Optellum collaborate for AI-based lung cancer diagnosis GE Healthcare has collaborated with Optellum to progress precision diagnosis and lung cancer treatment with artificial intelligence. The partnership is zeroed in on helping healthcare providers to evaluate the malignancy of a lung nodule a...
Read More...
Nov 25, 2021
Neurent Medical’s NEUROMARK; Brain Navi NaoTrac received European CE Mark approval; Theradaptive receives Breakthrough Medical Device Designation; HAGAR GWave awarded Breakthrough Device Designation; Fujifilm launches ColoAssist PRO
Neurent Medical obtains FDA Clearance for NEUROMARK™, a novel multi-point Nerve Disruption Treatment for Chronic Rhinitis On November 18, 2021, Neurent Medical, a company working in developing treatments for chronic inflammatory sino-nasal diseases, received clearance from the Food and Drug Ad...
Read More...
Nov 23, 2021
Bluebird bio may get gene therapy approved in the US; Neurocrine pens $2.6B pact with Sosei Heptares; FDA opens clearances for hepatitis C diagnostics; Twist nabs antibody discovery biotech Abveris for $190M
Bluebird bio may finally get a gene therapy approved in the US Bluebird bio may get gene therapy approved in the US. The Cambridge, MA biotech declared that the FDA had accepted and given priority review for Zynteglo, its gene therapy for the rare blood disorder beta-thalassemia. The announcement sets up an expe...
Read More...
Nov 18, 2021
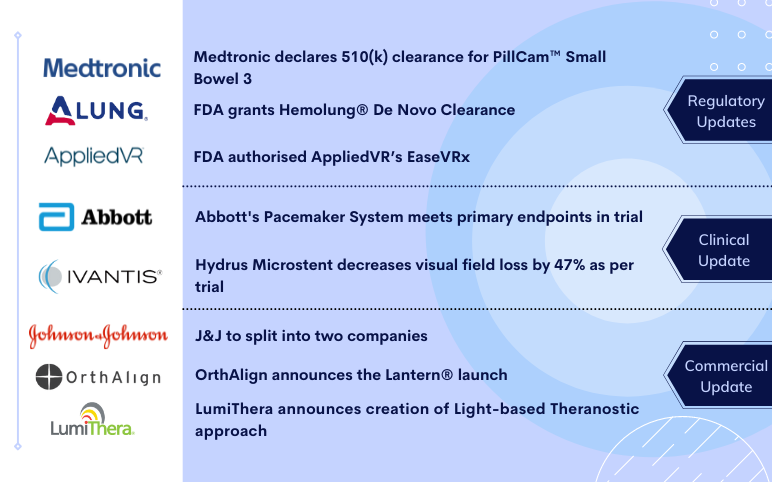
Abbott’s Pacemaker System; Ivantis’s Hydrus Microstent; Medtronic’s PillCam Small Bowel 3; ALung Technologies’s Hemolung; AppliedVR’s EaseVRx; J&J to split into two companies; OrthAlign’s Lantern® launch; LumiThera’s Light-based Theranostic
J&J intends to split into two companies, separating consumer products and pharmaceutical businesses On November 12, 2021, Johnson & Johnson announced its plans to split the Company’s Consumer Health business, forming a new publicly traded company. This will help the company create two global leaders tha...
Read More...
Nov 16, 2021
AstraZeneca-Moderna’s AZD8601 VEGF-A mRNA Trial; Wision AI gets CE Mark approval; Getinge acquires Verrix; LumiThera buys Diopsys
AstraZeneca, Moderna announces positive data of mRNA potential in heart failure patients Moderna, Inc., a biotechnology company is pioneering messenger RNA (mRNA) therapeutics and vaccines, declared positive data from the AstraZeneca-led Phase 2 (EPICCURE) study assessing the use of an mRNA therapeutic, wh...
Read More...
Nov 11, 2021
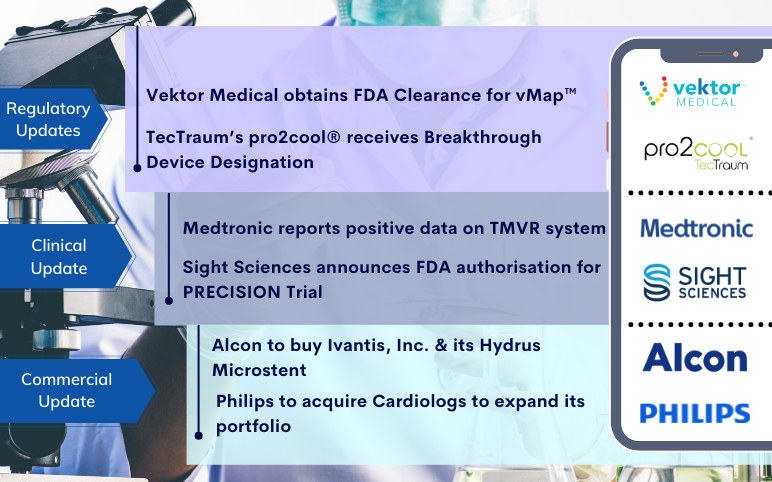
TecTraum’s pro2cool receives Breakthrough Device Designation; Vektor Obtains FDA Clearance for vMap; Medtronic’s TMVR system; Sight Sciences’s PRECISION Trial; Alcon to buy Ivantis; Philips to acquire Cardiologs
TecTraum’s pro2cool® receives FDA Breakthrough Device Designation for the Concussions treatment On November 03, 2021, TecTraum Inc., working to develop the world’s first point-of-care treatment for concussions, announced that the US Food and Drug Administration (FDA) has provided a Breakthrough Device to its f...
Read More...
Nov 09, 2021
Alcon to acquire Ivantis; Philips to takeover Cardiologs; Flunked cancer drug progresses in I-O in pancreatic cancer models; Indigo system CAT RX catheter meets primary endpoint in trial
Alcon to acquire Ivantis for USD 475 Million Alcon announced that it intends to buy glaucoma surgery device maker Ivantis for USD 475 million upfront. Geneva, Switzerland-based Alcon aims to support its portfolio across cataract, refractive, retina and glaucoma offerings by taking over Ivantis and its n...
Read More...
Nov 04, 2021
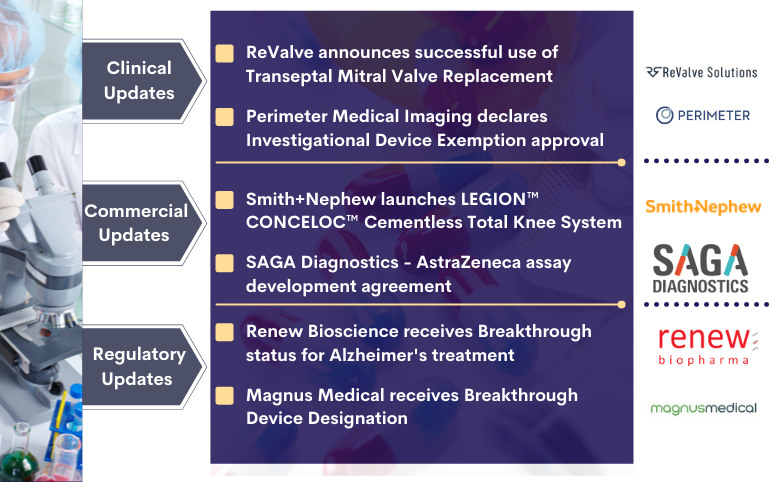
Smith+Nephew’s LEGION CONCELOC; Renew Bioscience’s Cerezen; Perimeter Medical’s ATLAS AI Project; SAGA Diagnostics-AZ’s Deal; Magnus Receives Breakthrough Device Designation; ReValve’s Transeptal Mitral Valve Replacement
ReValve Solutions, Inc. declares successful first human use of the Transeptal Mitral Valve Replacement On November 01, 2021, ReValve Solutions, Inc., a company operating in the field of transcatheter structural heart replacement and repair, announced the successful first human use of its transcatheter, Palmetto...
Read More...
Nov 02, 2021
Magnus Medical’s tech gets breakthrough status; Prolacta develops tests for pathogens detection; Renovia gets breakthrough designation for leva system; Hinge Health secures $600M funding
Magnus Medical’s neurostimulation technology gets FDA breakthrough status to treat MDD Magnus Medical has secured a breakthrough device designation from the US Food and Drug Administration (FDA) for its neurostimulation technology for major depressive disorder (MDD) treatment. The individualised, rapid-acting...
Read More...





-Agonist.png)

