news
Sep 23, 2021
Boston Scientific pays $336M; Boehringer Ingelheim acquires Abexxa Biologics; Oncology, RNA biotech 858 Therapeutics inks $60M Series A; Novartis acquires Arctos Medical
Boston Scientific pays USD 336 Million to acquire blood clot medical device firm Boston Scientific Corp. is shelling out as much as USD 336 million to take over the rest of medical device firm Devoro Medical, which it has held a stake in since 2019. The payment comprises USD 269 million paid upfront for the 8...
Read More...
Sep 21, 2021
Biogen’s AMD Biosimilar Gets Approval; SmartLabs Series B Funding; BARDA, Partner Therapeutics in Sepsis Space; Synlogic to Explore Sepsis Market Domain
Win-win for Biogen as its Biosimilar Gets Approval for AMD Samsung Bioepis and Biogen's biosimilar drug, Byooviz, received USFDA approval for the treatment of vision loss, Age-related macular degeneration and has become the first biosimilar to Lucentis in the AMD market. The Lucentis copycat had also manag...
Read More...
Sep 16, 2021
FDA’s New Drug Application to ALS Drug; Approval to BeiGene’s Brukinsa; Roche, Temedica Forges Digital Companionship, Shares of Sage Therapeutics Rises
FDA’s Go-Ahead to Amylyx New Drug Application for ALS Drug Amylyx Pharmaceuticals has announced its plan to submit a New Drug Application (NDA) to the U.S. FDA for its drug, AMX0035 (sodium phenylbutyrate (PB) and Taurursodiol (TURSO)), for the treatment of Amyotrophic lateral sclerosis (ALS). A f...
Read More...
Sep 14, 2021
ADARx Raises $75M Series B; Fast Track Designation for Vor Biopharma’s drug; Ixaka’s cell therapy clears phase 3; Breakthrough Device Designation to NovoTTF-200T™ System
ADARx bags USD 75 Million to advance its RNA tech pipeline ADARx Pharmaceuticals, a biotechnology company developing RNA targeting therapeutics, announced the completion of a USD 75 million Series B financing to progress its drug development pipeline. SR One Capital Management and OrbiMed Advisors co-led Series ...
Read More...
Sep 09, 2021
Roche inks $3B deal; Owlstone raises $58M; FDA approves OCS heart system; Google, Mayo Clinic build brain-mapping AI algorithm
Roche’s Genentech taps Adaptimmune for T-Cell Therapy Collaboration for USD 3 Billion A collaboration valued more than USD 3 billion, Genentech, a member of the Roche Group, declared it will collaborate with Adaptimmune for developing and commercializing allogeneic T-cell therapies to treat multiple cancer indic...
Read More...
Sep 07, 2021
Pfizer’s RSV Vaccine; Forte Biosciences’ Atopic Dermatitis Asset; Bone Therapeutics’ Osteoarthritis Programme; JandJ’s HIV Vaccine Trial; Versanis Bio $70 M Series A Financing; AC Immune’s Alzheimer’s Antibody Drug
Pfizer’s RSV Vaccine enters in the late-stage of clinical trials As the battle to get an effective Respiratory Syncytial Virus (RSV) vaccine on the market heats up, Pfizer is launching a critical late-stage study of its experimental vaccine against a severe version of a cold virus that may cause pneumonia ...
Read More...
Aug 31, 2021
Ascendis’s SKYTROFA; MicroTransponder’s VNS System (Vivistim); BioMarin’s Voxzogo; Zimmer-Canary’s Smart Knee Implant; COVID-19 impact on Solid Organ Transplants
FDA's Green Flag to Ascendis's Once-Weekly SKYTROFA for Treatment of Pediatric Growth Hormone Deficiency For the treatment of growth failure due to deficiency of growth hormones, the USFDA approved Ascendis Pharma's Skytrofa (lonapegsomatropin-tcgd) for children of one year and older suffering from an improper s...
Read More...
Aug 19, 2021
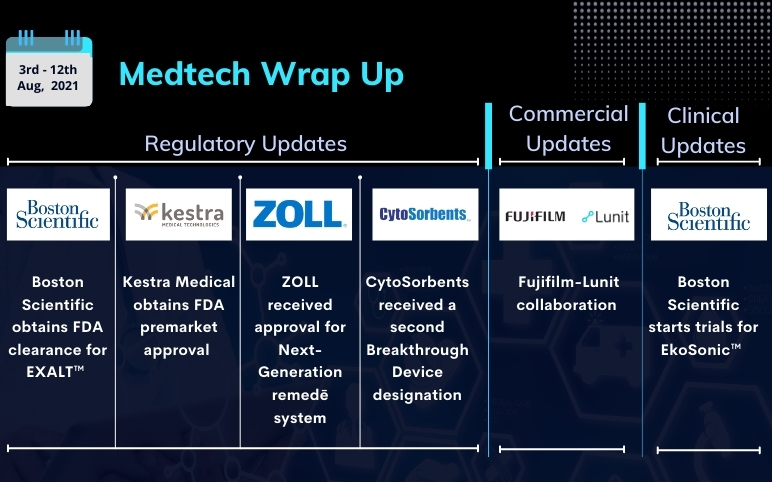
Boston Scientific’s EkoSonic; FDA clearance for EXALT; Kestra Medical’s ASSURE; ZOLL Next-Generation remedē system; CytoSorbents’ DrugSorb-ATR; Fujifilm-Lunit collaboration
Boston Scientific starts recruitment for randomized controlled trials for the EkoSonic™ Endovascular System On August 04, 2021, Boston Scientific Corporation started the enrolment in the HI-PEITHO clinical trial, which is a collaborative research study with the Pulmonary Embolism Response Team (PERT) Cons...
Read More...
Aug 17, 2021
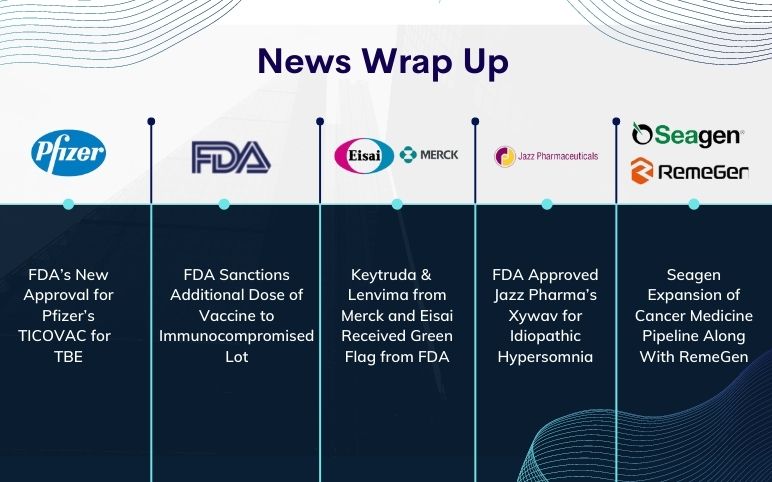
FDA approval to Keytruda and Lenvima; FibroGen’s Roxadustat; Pfizer’s TICOVAC; Ipsen’s Palovarotene; Jazz Pharma’s Xywav; Seagen-RemeGen’s Cancer Medicine
FDA’s Green Flag to Keytruda and Lenvima combination by Merck and Eisai for Advanced renal cell carcinoma (RCC) The FDA approved the combination of Keytruda and Lenvima produced by Merck and Eisai, as a first-line treatment of adult patients with advanced renal cell carcinoma (RCC). The efficacy and safety resul...
Read More...
Aug 12, 2021
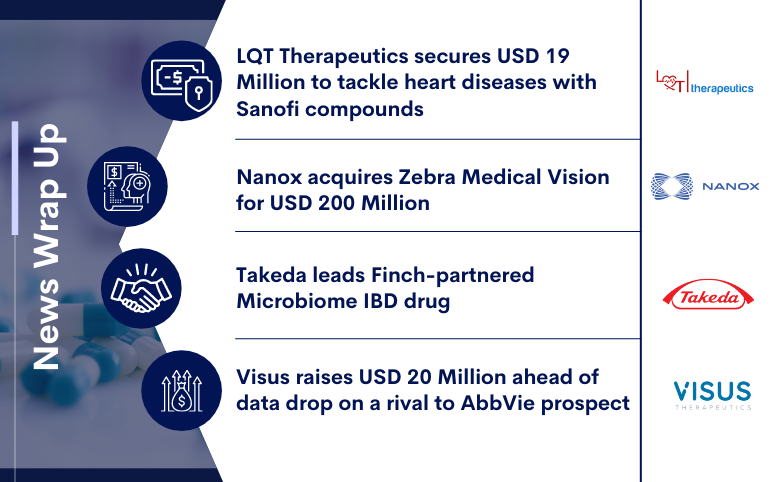
LQTT secures $19M; Nanox acquires Zebra Medical Vision; Takeda leads IBD drug; Visus raises $20M
LQT Therapeutics secures USD 19 Million to tackle heart diseases with Sanofi compounds LQT Therapeutics intends to tackle debilitating heart arrhythmias with limited interventions, and investors are planting a seed in the biotech's heart with USD 19 million. The two-year-old Canadian biotech will utiliz...
Read More...



-Agonist.png)

