news
Jun 11, 2024
Moderna’s Phase III Trials for Dual Influenza and COVID-19 Vaccine; Almirall’s Klisyri FDA Approval; Lilly’s Tirzepatide MASH Trial; Cycle Pharmaceuticals Acquisition of Vanda Pharmaceuticals; Ipsen’s Iqirvo FDA Approval
Moderna Reports Successful Phase III Trials for Dual Influenza and COVID-19 Vaccine Moderna, Inc. has reported that its Phase III trial for mRNA-1083, an experimental combination vaccine targeting both influenza and COVID-19, achieved its main objectives by generating a stronger immune response than the approved...
Read More...
Jun 06, 2024
Stryker’s LIFEPAK 35 Monitor/Defibrillator; Qiagen’s QIAstat-Dx Respiratory Panel; Evolution Optiks’s 510(k) Clearance; Microbot Medical Endovascular Surgical Robot Trial; Nexalin’s Insomnia and Anxiety Therapy Headset Clinical Trial; KORU Medical Systems’ Study With Commercialized Oncology Biologics
Stryker Released LIFEPAK 35 Monitor/Defibrillator On June 04, 2024, Stryker, a global leader in medical technologies, announced the launch of the LIFEPAK 35 monitor/defibrillator. This latest addition to their monitor/defibrillator lineup features advanced technology and is designed on an intuitive, modern...
Read More...
Jun 04, 2024
Moderna’s mRESVIA(R) FDA Approval; Novartis Scemblix® Phase III Data; Kite’s Tecartus for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia; Astellas’ Zolbetuximab Biologics License Application; CHMP Recommends AstraZeneca’s Tagrisso
FDA Greenlights Moderna's RSV Vaccine mRESVIA(R) Moderna, Inc. has announced that the FDA has approved mRESVIA (mRNA-1345), an mRNA-based vaccine for respiratory syncytial virus (RSV), to protect adults aged 60 and over from lower respiratory tract infections caused by RSV. This approval, granted under a breakth...
Read More...
May 28, 2024
AstraZeneca’s Tropion-Lung01 Phase III Trial Positive Outcomes; Biogen’s LEQEMBI South Korea Approval; AltruBio’s $225M Series B Funding; GSK’s Depemokimab Positive Phase III Severe Asthma Results; Roche’s Inavolisib FDA Breakthrough Therapy Designation
The Tropion-Lung01 Phase III Trial Reveals that Datopotamab Deruxtecan Significantly Enhanced Overall Survival in Advanced Nonsquamous NSCLC Patients Compared to Chemotherapy The high-level overall survival (OS) results from the TROPION-Lung01 Phase III trial, which had previously met the dual primary endpoint o...
Read More...
May 23, 2024
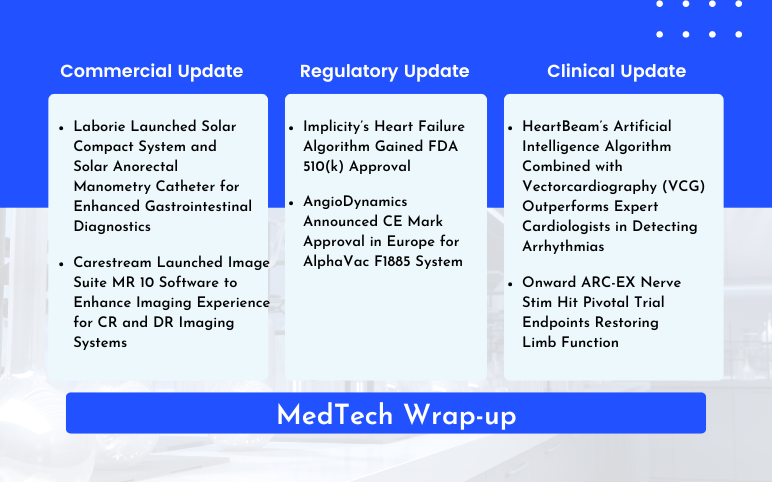
Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; Implicity’s Heart Failure Algorithm FDA 510(k) Approval; AngioDynamics’ AlphaVac F1885 System CE Mark Approval; HeartBeam’s Artificial Intelligence Algorithm for Arrhythmias; Onward ARC-EX Nerve Stim Hit Pivotal Trial
Laborie Launched Solar Compact System and Solar Anorectal Manometry Catheter for Enhanced Gastrointestinal Diagnostics On May 20, 2024, Laborie Medical Technologies Corp., launched the Solar Compact System and Solar Anorectal Manometry Catheter, the first disposable HRAM catheter on the market. These devices sig...
Read More...
May 21, 2024
Amgen’s IMDELLTRA FDA Approval; J&J’s Proteologix Acquisition; Bristol Myers Squibb’s BREYANZI FDA Approval; AbbVie and Gilgamesh Pharmaceuticals’ Agreement; Eisai’s LEQEMBI FDA Fast Track Status
IMDELLTRA Receives FDA Approval as the First T-Cell Engager Therapy for Advanced Small Cell Lung Cancer Amgen has reported that the FDA has approved IMDELLTRA™ (tarlatamab-dlle) for treating adult patients with extensive-stage small cell lung cancer (ES-SCLC) who have experienced disease progression following pl...
Read More...
May 14, 2024
Takeda and AC Immune’s Alzheimer’s Deal; Eli Lilly’s Donanemab FDA Review; Bristol Myers Squibb’s Phase III CheckMate -73L Trial Result; Regeneron Pharmaceuticals’ Dupixent sBLA; Excision BioTherapeutics’ EBT-101 Phase I/II Trial Results
Takeda and AC Immune Ink Exclusive Deal for Active Immunotherapy in Alzheimer’s, Focusing on Amyloid Beta Takeda and AC Immune SA have unveiled an exclusive global option and licensing pact concerning AC Immune’s active immunotherapies directed at harmful variants of amyloid beta (Abeta), notably ACI-24.060, int...
Read More...
May 09, 2024
Monteris Smallest Brain Laser Probe Launch; Vesica Health’s AssureMDx Test Launch; Anaut’s Eureka α Japanese Regulatory Approval; Geneoscopy’s Labcorp-Partnered Colon Cancer Test FDA Approval; Novel Omeza® OCM™ Diabetic Foot Ulcers Results; LEO Pharma Phase III Plaque Psoriasis Trial Results
Monteris Launched Smallest Brain Laser Probe on the Market On May 01, 2024, Monteris Medical launched the NeuroBlate® NB3™ FullFire® 1.6mm laser probe, the company’s latest product line innovation for use with their market-leading NeuroBlate System. The NB3 laser probe, which integrates Monteris' patented coo...
Read More...
May 07, 2024
Boehringer Ingelheim’s Diabetic Macular Ischemia Study; Novartis’ Mariana Oncology Acquisition; Astellas and Poseida Therapeutics Partnership; Prime Medicine’s PM359 IND; Pfizer’s TIVDAK FDA Approval
Boehringer Ingelheim Announces Promising Findings From Groundbreaking Study on Diabetic Macular Ischemia Boehringer Ingelheim released encouraging results from the HORNBILL Phase I/IIa trial of BI 764524, marking the pioneering investigation into a potential therapy for individuals with diabetic macular is...
Read More...
Apr 30, 2024
X4 Pharmaceuticals’ XOLREMDI FDA Approval; ONO to Acquire Deciphera Pharmaceuticals; Johnson & Johnson’s SIRTURO CHMP Approval; BeiGene’s Tislelizumab EC Approval; Sanofi’s Rilzabrutinib LUNA 3 Phase III Trial
FDA Greenlights XOLREMDI Capsules from X4 Pharmaceuticals for WHIM Syndrome Treatment X4 Pharmaceuticals has declared that the FDA has granted approval for XOLREMDI™ (mavorixafor) capsules to be utilized in individuals aged 12 and above who have WHIM syndrome (characterized by warts, hypogammaglobulinemia, infec...
Read More...









-Agonist.png)

