NICE
Apr 11, 2023
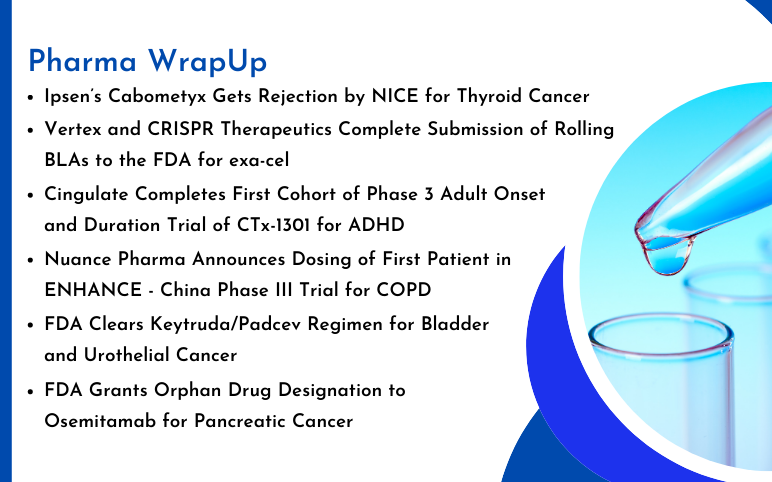
Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for exa-cel; Orphan Drug Designation to Osemitamab for Pancreatic Cancer; FDA Clears Keytruda/Padcev for Bladder and Urothelial Cancer; Cingulate Completes Trial of CTx-1301 for ADHD; Nuance Pharma Announces Dosing of First Patient in ENHANCE Trial
FDA Grants Orphan Drug Designation to Osemitamab for Pancreatic Cancer Transcenta Holding Limited has announced that the U.S. Food and Drug Administration (FDA) has awarded Orphan Drug Designation to Osemitamab (TST001), a highly potent humanized monoclonal antibody that enhances ADCC (antibody-dependent cell-me...
Read More...
Apr 17, 2017
Whitepaper: Impact of Brexit on Pharma Space
BREXIT shook the whole world when United Kingdom voted in favour of leaving the EU. After the vote, the country has been wrought in uncertainty related to political, regulatory and economic scenario. Brexit has led to deliberations regarding modified supply chain requirements, uncertain regulatory scenario, revised ...
Read More...

-Agonist.png)

