Non-small cell lung cancer
Oct 15, 2024
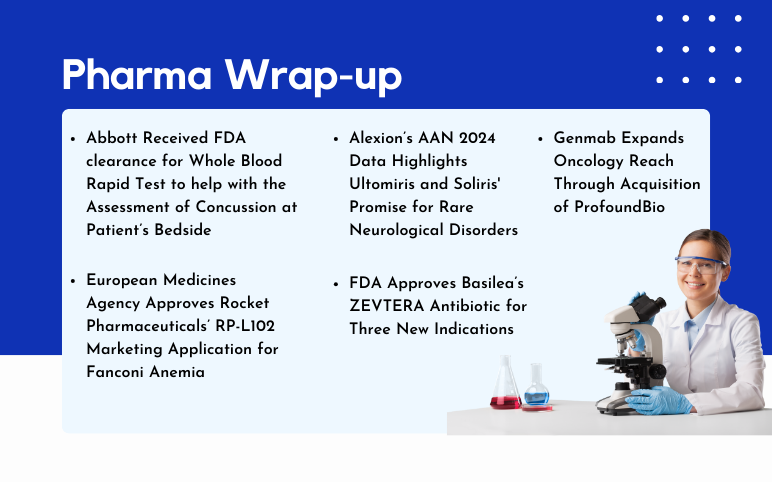
Astellas & AviadoBio’s Exclusive Deal for AVB-101; GSK’s Depemokimab Shows Positive Results for Nasal Polyps; Lilly’s Mirikizumab Outperforms Ustekinumab in Crohn’s Study; TREMFYA Delivers Strong Results in Crohn’s & Colitis; ENHERTU Approved in China for HER2-Mutant NSCLC.
Astellas & AviadoBio Sign Exclusive Deal for Gene Therapy AVB-101 in Frontotemporal Dementia AviadoBio Ltd. and Astellas Pharma Inc. have announced a strategic partnership under an exclusive option and license agreement for AVB-101, an investigational AAV-based gene therapy currently in Phase I/II developmen...
Read More...
Aug 27, 2024
Merck’s WINREVAIR™ EU Approval; BALVERSA for Urothelial Carcinoma; Novartis and Versant’s Borealis Launched; Moderna’s RSV mRESVIA® EU Approval; FDA Nods RYBREVANT® and LAZCLUZE™ for EGFR-mutated Lung Cancer
Merck’s WINREVAIR Approved by the European Commission for PAH in Adults with Functional Class II-III Merck has secured European Commission (EC) approval for WINREVAIR™ (sotatercept), marking it as the first activin signaling inhibitor therapy for pulmonary arterial hypertension (PAH) approved across all 27 EU me...
Read More...
Aug 20, 2024
Gilead’s Livdelzi FDA Approval for Primary Biliary Cholangitis; Incyte and Syndax’s Niktimvo Approved for Graft-Versus-Host Disease; FDA Lifts Hold on BioNTech and MediLink’s ADC Cancer Trial; FDA Approves IMFINZI for Resectable Lung Cancer; Yutrepia Receives Tentative Approval for PAH and PH-ILD
Gilead’s Livdelzi (Seladelpar) Granted Accelerated Approval for Primary Biliary Cholangitis by FDA Gilead Sciences, Inc. has received accelerated approval from the FDA for Livdelzi® (seladelpar) in the treatment of primary biliary cholangitis (PBC). Livdelzi can be used in combination with ursodeoxycholic acid (...
Read More...
Jul 09, 2024
Lilly’s Morphic Acquisition; IDEAYA’s IDE397 Positive Phase II Trial Result; XPOVIO (selinexor) Approval in China; Roche to Reintroduce Susvimo in the US; Dupixent EU Approval
Lilly Strengthens IBD Treatment Portfolio with Morphic Acquisition Eli Lilly and Company and Morphic Holding, Inc. announced a definitive agreement for Lilly to acquire Morphic, a biopharmaceutical company developing oral integrin therapies for serious chronic diseases. Lilly will initiate a tender offer to acqu...
Read More...
Apr 15, 2024
ENHERTU: Another Triumph to Celebrate for AstraZeneca and Daiichi Sankyo
ENHERTU lies at the core of AstraZeneca and Daiichi Sankyo’s objectives for advancing in oncology. This collaboration has notably broadened the antibody-drug conjugates impact in the United States. On April 5, 2024, the FDA approved ENHERTU to treat HER2-positive solid tumors in adults who have received previous sy...
Read More...
Apr 09, 2024
AstraZeneca and Daiichi Sankyo’s Enhertu US Approval; Basilea’s ZEVTERA FDA Approval; Genmab’s ProfoundBio Acquisition; Rocket Pharmaceuticals’ RP-L102 EMA Approval; Alexion’s Ultomiris and Soliris AAN 2024 Data
Enhertu Receives US Approval as First HER2-focused Treatment for Metastatic Solid Tumors, Independent of Tumor Origin AstraZeneca and Daiichi Sankyo's drug Enhertu (trastuzumab deruxtecan) has gained approval in the United States for treating adult patients with inoperable or metastatic HER2-positive (IHC 3+) so...
Read More...
Mar 15, 2024
Unveiling the Potential of TROP-2 Inhibitors: A New Frontier in Cancer Treatment
Trophoblast cell surface antigen-2 (TROP-2) is a 35-kDa protein found on cell membranes, made of sugars and a protein chain, serving as a transmitter of calcium signals. It is produced by the TACSTD2 gene and shares a structural similarity with the epithelial cell adhesion molecule (EpCAM). TROP2, although ident...
Read More...
Mar 05, 2024
Bayer’s New Cardiology Drug Acoramidis; Two Datopotamab Deruxtecan Applications Validated in the EU; AbbVie and OSE Immunotherapeutics Announce Announces Partnership; vTv Therapeutics Makes Major Move With Cadisegliatin; A2 Bio Scores FDA Orphan Drug Designation for its Therapy, A2B530; FDA Fast Track Designation for AlloNK® in Lupus Nephritis
Acoramidis Joins Bayer's Robust Lineup, Boosting Cardiology Solutions Bayer has obtained the exclusive rights to market acoramidis in Europe from Eidos Therapeutics Inc., BridgeBio International GmbH, and BridgeBio Europe B.V. Acoramidis, a highly potent and selective small molecule given orally, functions as a ...
Read More...
Nov 27, 2023
Another Feather in the Cap for Xtandi and Keytruda — The Two Main Cancer Drugs
The FDA has approved label extensions for two of the most crucial cancer medications globally—Merck’s Keytruda and Pfizer and Astellas’ Xtandi. Keytruda’s expanded indication now includes stomach cancer, permitting its usage alongside chemotherapy for first-line treatment in patients with locally advanced unresecta...
Read More...
Sep 08, 2023
A Quick Review of Antibody-Drug Conjugates (ADCs) in Lung Cancer: HER2, TROP-2, HER3, MET, and CEACAM5 ADC Advancements
Lung cancer remains the leading cause of cancer-related death in the United States. The American Cancer Society estimates 238,340 people will be diagnosed with lung cancer in 2023, with non-small cell lung cancer (NSCLC) accounting for the majority of cases (roughly 80% to 85%).The treatment paradigm of antibody-dr...
Read More...






-Agonist.png)

