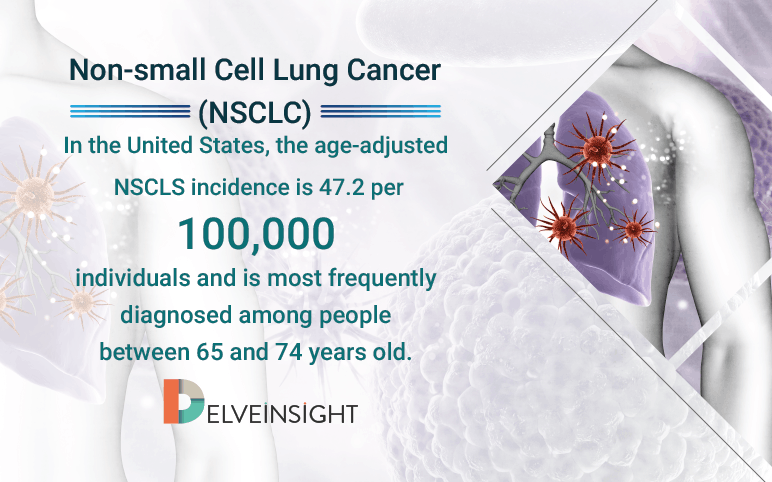
NSCLC
Nov 01, 2021
An in-depth Assessment of the Top Drugs Launched by Leading Global Companies in the First Half of 2021 (H1)
Progress is driven by innovation. When it comes to developing novel medications and therapeutic biological products, the FDA's Center for Drug Evaluation and Research (CDER) assists the pharmaceutical sector at every stage of the process. CDER offers scientific and regulatory assistance needed to bring innovative m...
Read More...
Jun 01, 2021
Amgen a leader in Undruggable Lung Cancer; Verrica’s Skin Disease Drug Delays; Fennec’s Faith in its Chemotherapy-Induced Hearing Loss Drug; Nexturn Bio’s Acquisition of RosVivo
FDA Validates Use of Amgen’s KRAS Inhibitor in Lung Cancer In a pathbreaking move, Amgen has gone ahead to emerge as a trailblazer in the undruggable lung cancer space with the approval of its KRAS inhibitor, Lumakras. For eons, the researchers grappled to come up with an effective and potential drug to ta...
Read More...
Mar 23, 2021
Evotec, Takeda Deal; AZ COVID Vaccine US Trials; Promega’s Recognition; Roche’s Tecentriq Results in NSCLC
Promega Biotech Ibérica Gains Recognition for COVID-19 Response in Spain Promega Biotech Ibérica has been heralded as the business leader in Spain for its COVID-19 response. The company’s innovative approach to deal with COVID-19 and pandemic has led La Razón, a daily newspaper based in Madrid, to felicitate the...
Read More...
Feb 23, 2021
Clover biopharmaceuticals raises $230 M; BrainStorm after ALS; BARDA grants $5.65 M LightDeck for SARS-CoV-2 antigen test; Libtayo for first-line advanced NSCLC subset
Clover Biopharmaceuticals Raises $230 Million Clover Biopharmaceuticals announced the completion of an oversubscribed $230 million Series C financing, summing total capital raised by the company during the last year over USD 400 million. The Series C round was co-led by GL Ventures and Temasek, with partic...
Read More...
Jul 28, 2020
AZ, Daiichi inks an oncology deal; Moderna begins Phase III trial of its COVID-19 vaccine; No good news for Solid’s DMD gene therapy trial
AZ, Daiichi put their trust into TROP2-based drugs, inks an oncology deal to together develop and commercialize DS-1062 AstraZeneca has inked an oncology deal with Daiichi Sankyo to jointly develop and commercialize an antibody-drug conjugate DS-1062 (trastuzumab deruxtecan), worldwide except in Japan. Daiichi ...
Read More...
Jul 01, 2020
Top 5 Cancers Creating Major Challenge To The Global Healthcare System
Over the past few decades Cancer has emerged as a major public health concern worldwide. Cancer is the second leading cause of deaths that takes place worldwide. Every sixth death is due to cancer, and in 2018 was estimated to be responsible for 9.6 million deaths. Costing people quality of lives and eventually lea...
Read More...
Dec 05, 2019
Reblozyl under FDA review; Audentes buyout & Tecentriq’s new approval
Bristol-Meyers Squibb and Acceleron Pharma have announced that US FDA committee will review BMS’s supplemental Biologics License Application (sBLA) for its Reblozyl in MDS. Reblozyl ((luspatercept-aamt) has recently been approved by the regulatory authority to cure a rare type of blood disorder, Beta-thalassemi...
Read More...
Aug 23, 2019
Imfinzi fails. What’s next in Non-small cell lung cancers Market Scenario?
The drug Imfinzi (durvalumab) has failed to come up as an effective treatment option for treating non-small cell lung cancer (NSCLC). The result of the NEPTUNE study was not much of a promising one and has been disclosed completely. The Pharma giant, AstraZeneca, has announced the incompetence of its lead candidate...
Read More...
Oct 11, 2018
Copiktra receives approval; Lilly wins approval; Eisai’s Fycompa; Astellas’ Roxadustat; Janssen’s Esketamine Nasal Spray
SNAPSHOTS: Copiktra receives FDA approval for CLL and FL, Relief for tazemetostat developers after FDA lifted partial clinical hold, Conference highlights from 19th WCLC The United States Food and Drug Administration (USFDA) has granted approval to Verastem’s Copiktra (duvelisib), for the treatment of patients with...
Read More...
Nov 03, 2017
AstraZeneca and Incyte Collaborate; Tocagen’s therapy; FDA warns companies;FDA Revokes
AstraZeneca and Incyte are about to enter into Clinical Trial Collaboration in Early Lung Cancer The exclusive collaboration for the study population allows the two companies to conduct a Phase 3 trial. Aim of this study is to evaluate patients with locally-advanced (Stage III), unresectable non-small cell lung c...
Read More...








-Agonist.png)

