Ophthalmic Devices
Jun 27, 2024
Alcon’s 510(k) Clearance From the FDA; Globus Medical Received FDA Clearance; UK Pioneers First Skull-Mounted Epilepsy Device for Child; The Initiation of a Significant Clinical Trial of 3d Printed Models; Esaote Unveiled the Cutting-Edge Mylab™E80 @Sirm Ultrasound Device For 2024; Medasense Unveiled a Transformative Partnership With Nihon Kohden Corporation
Alcon's Newest Technological Marvels, Unity VCS, and Unity CS, Achieved 510(k) Clearance From the U.S. FDA, Paving the Way for New Advancements On June 24, 2024, Alcon, the foremost name in eye care with a mission to help people see brilliantly, revealed that the U.S. Food and Drug Administration (FDA) had...
Read More...
Apr 11, 2024
Carl Zeiss Meditec AG’s Acquisition of Dutch Ophthalmic Research Center; Johnson & Johnson’s Shockwave Medical Acquisition; Biora Therapeutics’ BT-600 Positive Results; Medtronic’s Single-Shot Mapping Ablation Catheter Positive Data; Abbott’s TriClip FDA Approval; Abbott’s Whole Blood Rapid Test FDA Clearance
Carl Zeiss Meditec AG Completed Acquisition of Dutch Ophthalmic Research Center (D.O.R.C); Companies Unite to Shape Ophthalmology Market On April 04, 2024, Carl Zeiss Meditec AG announced that it acquired D.O.R.C. (Dutch Ophthalmic Research Center) from the investment firm Eurazeo SE, Paris, France. T...
Read More...
Dec 07, 2023
Johnson & Johnson Medtech Acquired Laminar; BD Launched Advanced Vascular Access Ultrasound System; FDA Cleared Oral Device for Severe Sleep Apnea; FDA Clearanced the Zilia OcularTM FC Retinal Camera; First Patient Enrolled in Penumbra Study of Computer-assisted Vacuum Thrombectomy; US FDA Granted the BiVACOR Total Artificial Heart IDE Approval for First-in-Human Early Feasibility Study
FDA Cleared Oral Device for Severe Sleep Apnea From Vivos Therapeutics On November 29, 2023, Vivos Therapeutics announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Vivos’s removable CARE (Complete Airway Repositioning and Expansion) oral appliances developed for treat...
Read More...
Jun 30, 2022
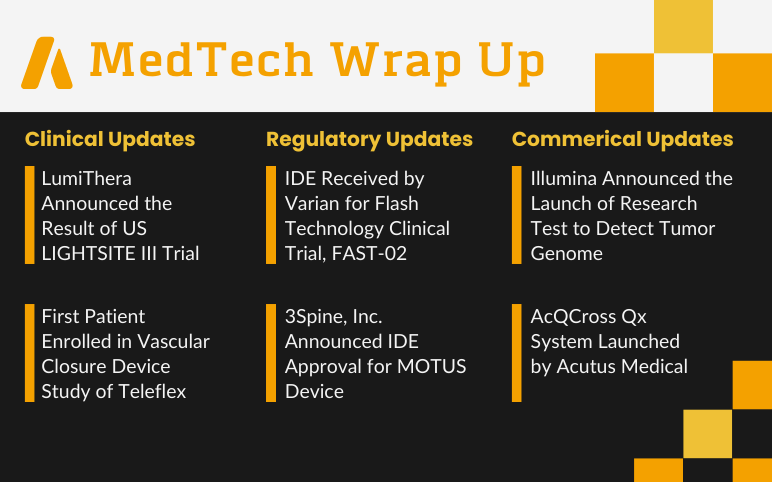
LumiThera’s US LIGHTSITE III Trial; First Patient Enrolled in Vascular Closure Device Study of Teleflex; Varian’s Flash Technology Clinical Trial, FAST-02; 3Spine’s MOTUS Device; Illumina’s Research Test to Detect Tumor Genome; Acutus Medical’s AcQCross Qx System
LumiThera Announced the Result of US LIGHTSITE III Trial of Non-neovascular Age-Related Macular Degeneration (AMD) Subjects Treated with Photobiomodulation (PBM) using the Valeda® Light Delivery System On June 22, 2022, LumiThera Inc., a commercial-stage medical device company offering photobiomodulation (PBM) t...
Read More...



-Agonist.png)

