Pancreatic Cancer
Nov 11, 2024
5 Novel CDK7 Inhibitors Pushing the Boundaries in Oncology
In the rapidly evolving field of oncology, novel CDK7 inhibitors are emerging as game-changers, challenging the status quo of cancer treatment. By specifically targeting CDK7, these innovative therapies not only halt cancer cell proliferation but also enhance the effectiveness of existing treatments. With the abili...
Read More...
Oct 02, 2024
Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies
With millions of cases diagnosed globally each year, gastrointestinal cancers represent a significant health challenge but also an opportunity for breakthroughs in targeted therapies and personalized medicine. Gastrointestinal cancers refer to a group of cancers that affect the digestive system, including the esoph...
Read More...
Jul 01, 2024
Pancreatic Cancer Treatment: Top 8 Emerging Therapies to Watch Out
Pancreatic cancer is enlisted as one of the most common cancers and the seventh-highest cause of cancer mortality worldwide. The incidence of pancreatic cancer is increasing in the Western world. The incidence of pancreatic adenocarcinoma is rising in the developed world, and modifiable lifestyle factors such as al...
Read More...
Feb 23, 2024
Ipsen’s Onivyde Combo Ends a Decade-Long Dry Spell in Newly Diagnosed Pancreatic Cancer
It marks the awaited green light for Ipsen following its acquisition of Onivyde in 2017. The FDA has given its nod to the supplemental new drug application for Onivyde (irinotecan liposome injection) in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) as a primary treatment for adults with met...
Read More...
Dec 26, 2023
ImPact Biotech’s IND Application for Padeliporfin VTP; Orphan Drug Designation to Ocelot Bio’s OCE-205 for Ascites; Amylyx’s Phase 3 ORION Study of AMX0035 for PSP; SELLAS Receives FDA Orphan Drug Designation for SLS009 for PTCL Treatment; Apnimed Updated on Second Phase 3 Clinical Study of AD109 for OSA
ImPact Biotech Receives FDA Clearance of IND Application for Padeliporfin VTP in Pancreatic Cancer ImPact Biotech, a biotechnology company in its clinical stage dedicated to advancing Padeliporfin Vascular Targeted Photodynamic (VTP) therapy for various solid tumors, announced on December 20, 2023, that the U.S....
Read More...
Oct 24, 2023
FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved Moderate to Severe Plaque Psoriasis; FDA Approves BioMarin’s VOXZOGO; FDA Fast Track Designation to ANPD001 for Parkinson’s Disease; UCB Announces FDA Approval of ZILBRYSQ; EMA Granted Orphan Drug Designation to Lisata’s LSTA1
UCB announces FDA approval of ZILBRYSQ for the Treatment of Adults with Generalized Myasthenia Gravis On the 17th of October 2023, UCB (Euronext Brussels: UCB) made an announcement regarding the approval of ZILBRYSQ® (zilucoplan) by the US FDA for the management of generalized myasthenia gravis (gMG) in adult pa...
Read More...
Aug 22, 2023
Eylea HD Injection 8 Mg Approved By FDA; Veopoz Receives FDA Approval for CHAPLE Disease Treatment; FDA Places Second Partial Clinical Hold on AML Enrollment for Magrolimab Trials; FDA Approval to Incannex’s Sleep Apnoea Clinical Trial; FDA Orphan Drug Designation to Avidity’s AOC 1044; Orphan Drug Designation to CanariaBio’s MAb-AR20.5
Eylea HD Injection 8 Mg Approved By FDA for Treatment of Wet AMD, DME, and Diabetic Retinopathy The FDA has approved Regeneron Pharmaceuticals’ EYLEA HD (aflibercept) Injection of 8 mg for the treatment of patients with wet age-related macular degeneration (wAMD), diabetic macular edema (DME), and diabetic retin...
Read More...
May 23, 2023
FDA Approves RINVOQ for Crohn’s Disease; FDA Approves Krystal Biotech’s Gene Therapy Vyjuvek; FDA Approves EPKINLY to Treat R/R DLBCL; FDA Orphan Drug Designation to Mitazalimab; Phase 3 Trial Result of OCS-01 Eye Drops; TAGRISSO® + Chemotherapy for the EGFR-mutated Advanced Lung Cancer
FDA Approves RINVOQ as a Once-Daily Pill for Moderately to Severely Active Crohn's Disease AbbVie announced that the FDA had approved RINVOQ® (upadacitinib) for treating people with moderately to highly active Crohn's disease who have had an unsatisfactory response or intolerance to one or more TNF blockers. Thi...
Read More...
Apr 11, 2023
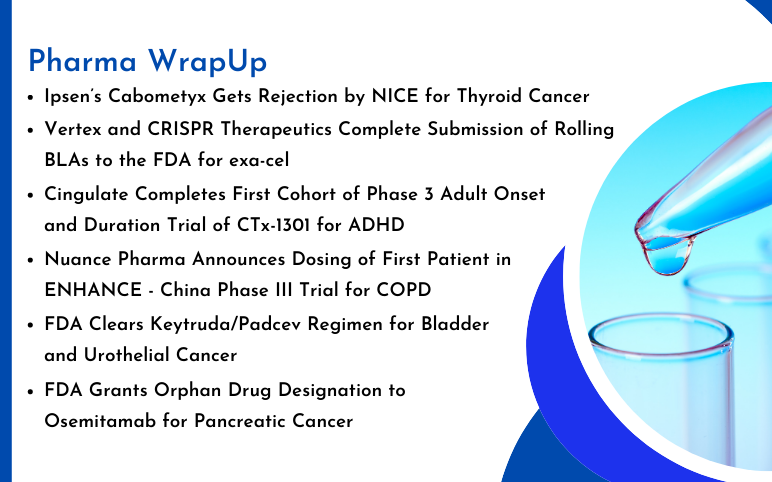
Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for exa-cel; Orphan Drug Designation to Osemitamab for Pancreatic Cancer; FDA Clears Keytruda/Padcev for Bladder and Urothelial Cancer; Cingulate Completes Trial of CTx-1301 for ADHD; Nuance Pharma Announces Dosing of First Patient in ENHANCE Trial
FDA Grants Orphan Drug Designation to Osemitamab for Pancreatic Cancer Transcenta Holding Limited has announced that the U.S. Food and Drug Administration (FDA) has awarded Orphan Drug Designation to Osemitamab (TST001), a highly potent humanized monoclonal antibody that enhances ADCC (antibody-dependent cell-me...
Read More...
Sep 20, 2022
AstraZeneca’s Danicopan Trial; CHMP Recommends Sanofi/AstraZeneca’s nirsevimab; Akero’s NASH Drug Trial; FDA Grants Orphan Drug Status to SY-5609; BMS’s Opdivo Trial Results; Pfizer to File for FDA Approval for Meningitis Vaccine; EMA Orphan Drug Designation to CAN-2409; FDA Starts Priority Review of Chiesi ‘s velmanase alfa
AstraZeneca’s Danicopan Shows Positive Results in Phase III Trial Danicopan, an oral Factor D inhibitor developed by AstraZeneca, was expected to fail a phase II trial in rare kidney disease in 2020, but a new readout could revive the drug. Danicopan (ALXN2040) has demonstrated efficacy as an adjunct treatment f...
Read More...






-Agonist.png)

