PET-MRI Systems
Jan 23, 2025
Tempus Launches FDA-Approved xT CDx Test Nationwide; B. Braun Enhances Catheter Securement with the Launch of Clik-FIX; Illuccix® Secures European Regulatory Approval; FDA Clears CapsoCam Plus® for Remote Ingestion; BellaSeno Marks Success in Two Clinical Trials for Revolutionary Breast Implant Technology; AF Symposium to Feature Conformal Medical’s Groundbreaking GLACE Study
Tempus Announced the National Launch of the FDA-Approved xT CDx Test On January 15, 2025, Tempus AI, Inc., a technology company driving the use of AI to advance precision medicine and improve patient care, announced the nationwide launch of its FDA-approved, NGS-based in vitro diagnostic device, xT CDx. Th...
Read More...
Nov 14, 2024
FDA Grants Approval to Caris Life Sciences for MI Cancer Seek; Johnson & Johnson MedTech Secures FDA IDE Approval for OTTAVA; Organogenesis Shares Positive Interim Data from Second Phase 3 Study of ReNu; LivaNova’s OSPREY Trial Achieves Key Safety and Efficacy Milestones; Hyperfine Launches Advanced Portable MR Brain Imaging Swoop® System Across Europe; Nihon Kohden Strengthens Neurological Solutions Through Ad-Tech Medical Instrument Corporation Acquisition
Caris Life Sciences Received FDA Approval for MI Cancer Seek™ as a Companion Diagnostic (CDx) Test On November 6, 2024, Caris Life Sciences, a leading next-generation AI TechBio company and precision medicine pioneer, announced that the U.S. Food and Drug Administration (FDA) approved MI Cancer Seek™ for u...
Read More...
May 16, 2024
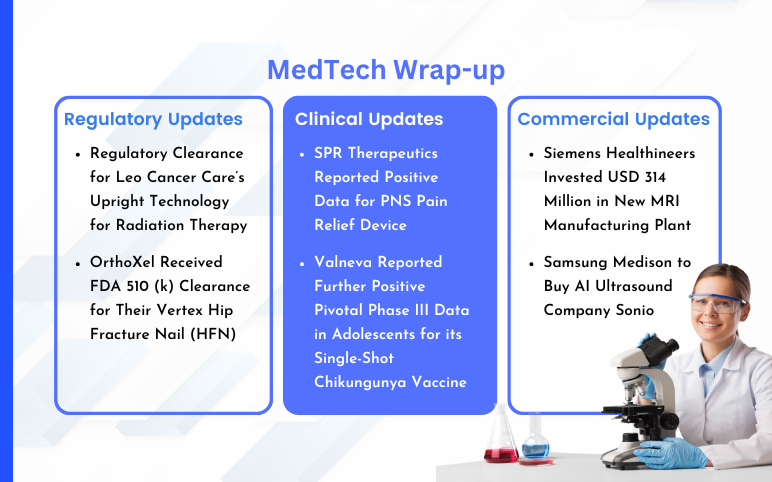
Siemens Healthineers USD 314 Million Investment; Samsung Medison’s Sonio Acquisition; Leo Cancer Care’s Upright Technology Clearance; OrthoXel’s Vertex Hip Fracture Nail FDA 510 (k) Clearance; SPR Therapeutics’ PNS Pain Relief Device Positive Data; Valneva’s Chikungunya Vaccine Positive Pivotal Phase III Data
Siemens Healthineers Invested USD 314 Million in New MRI Manufacturing Plant On May 15, 2024, Siemens Healthineers announced that it had invested USD 314 million in a new MRI manufacturing plant in the UK. The facility in Oxford, England, which Siemens anticipates opening in 2026, will develop technology to redu...
Read More...


-Agonist.png)

