pharma news
Apr 16, 2024
Roche’s Columvi Phase III STARGLO Trial; Novartis’ Fabhalta Latest Data; Vertex’s Alpine Immune Sciences Acquisition; Telix Pharmaceuticals’ TLX101-CDx Fast Track Designation; NovelMed’s NM5072 Orphan Drug Designation
Roche's Columvi Achieves Primary Endpoint of Prolonged Overall Survival in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma in Phase III STARGLO Trial Roche reported that the Phase III STARGLO trial successfully achieved its main goal of improving overall survival. The research revealed that in...
Read More...
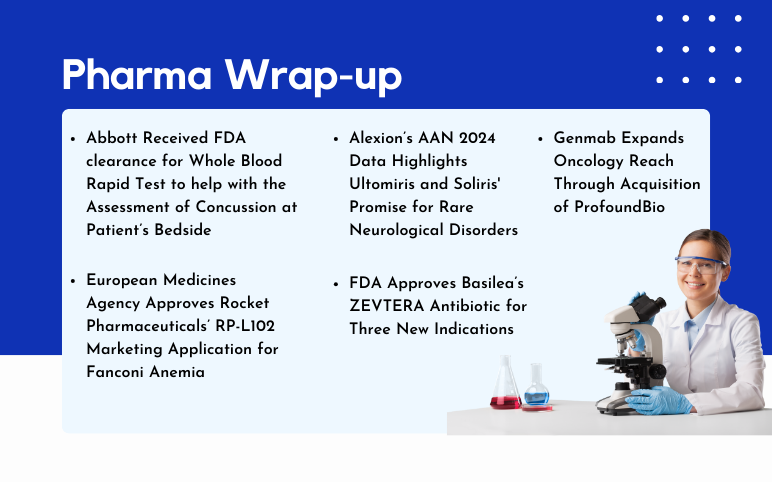
Apr 09, 2024
AstraZeneca and Daiichi Sankyo’s Enhertu US Approval; Basilea’s ZEVTERA FDA Approval; Genmab’s ProfoundBio Acquisition; Rocket Pharmaceuticals’ RP-L102 EMA Approval; Alexion’s Ultomiris and Soliris AAN 2024 Data
Enhertu Receives US Approval as First HER2-focused Treatment for Metastatic Solid Tumors, Independent of Tumor Origin AstraZeneca and Daiichi Sankyo's drug Enhertu (trastuzumab deruxtecan) has gained approval in the United States for treating adult patients with inoperable or metastatic HER2-positive (IHC 3+) so...
Read More...
Apr 02, 2024
AstraZeneca’s Voydeya FDA Approval; Akebia’s Vafseo FDA Approval; Bristol Myers Squibb’s Phase III YELLOWSTONE Trial Update; Astellas’ IZERVAY FDA Approval; AstraZeneca’s Truqap and Faslodex MHLW Approval
Voydeya Receives FDA Approval as Supplemental Treatment with Ravulizumab or Eculizumab for Managing Extravascular Hemolysis in Adult Patients with PNH Voydeya (danicopan) has received approval in the United States for use alongside ravulizumab or eculizumab in treating extravascular hemolysis (EVH) in adults dia...
Read More...
Mar 26, 2024
Regeneron’s Odronextamab BLA; Novo Nordisk’s Cardior Pharmaceuticals Acquisition; Novartis’ Fabhalta CHMP Approval; Idorsia’s TRYVIO FDA Approval; AbbVie’s Landos Biopharma Acquisition
Regeneron Updates Progress on Biologics License Application for Odronextamab Regeneron Pharmaceuticals, Inc. has announced that the FDA has issued Complete Response Letters (CRLs) regarding the Biologics License Application (BLA) for odronextamab in cases of relapsed/refractory (R/R) follicular lymphoma (FL) and...
Read More...
Mar 05, 2024
Bayer’s New Cardiology Drug Acoramidis; Two Datopotamab Deruxtecan Applications Validated in the EU; AbbVie and OSE Immunotherapeutics Announce Announces Partnership; vTv Therapeutics Makes Major Move With Cadisegliatin; A2 Bio Scores FDA Orphan Drug Designation for its Therapy, A2B530; FDA Fast Track Designation for AlloNK® in Lupus Nephritis
Acoramidis Joins Bayer's Robust Lineup, Boosting Cardiology Solutions Bayer has obtained the exclusive rights to market acoramidis in Europe from Eidos Therapeutics Inc., BridgeBio International GmbH, and BridgeBio Europe B.V. Acoramidis, a highly potent and selective small molecule given orally, functions as a ...
Read More...
Feb 20, 2024
FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas and Kelonia Enter into Research and License Agreement; Fast Track Designation to Certa’s FT011; Innovent Announces Phase 3 Clinical Trial Updates for IBI311; Orphan Drug Designation to Cardiol’s Pericarditis Drug Candidate
FDA Approves Xolair as First and Only Medicine for Children and Adults with One or More Food Allergies Roche has announced that the FDA has approved Xolair® (omalizumab) to mitigate allergic responses, such as anaphylaxis, that may arise from accidental exposure to various foods in both adult and pediatric patie...
Read More...
Feb 13, 2024
GSK Receives FDA Fast Track Designation for Bepirovirsen; Gilead to Acquire CymaBay Therapeutics; CSL Announces Top-line Results from the Phase III AEGIS-II Trial; Ruxoprubart Scores FDA Orphan Drug Designation for PNH Treatment; CymaBay Announces FDA Acceptance of NDA and Priority Review for Seladelpar; Biogen Received European Commission Approval for SKYCLARYS
GSK Receives FDA Fast Track Designation for Bepirovirsen in Chronic Hepatitis B GSK plc has revealed that the US Food and Drug Administration (FDA) has awarded Fast Track status to bepirovirsen, an experimental antisense oligonucleotide (ASO) designed to treat chronic hepatitis B (CHB). Fast Track designation ai...
Read More...
Feb 07, 2024
Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
As we step into the crisp corridors of 2024, the healthcare landscape unfolds a compelling saga of mergers, strategic funding, and transformative acquisitions. In this month-by-month analysis, we delve into the intricate tapestry of industry dynamics, exploring the impactful maneuvers that are shaping the healthcar...
Read More...
Feb 06, 2024
4DMT Presents Data From Phase II PRISM Clinical Trial; Adaptimmune Announces FDA Acceptance of Biologics License Application for Afami-cel; Astellas Submits Supplemental New Drug Application in Japan for PADCEV with KEYTRUDA; AnaMar Announces US and EU Orphan Drug Designation for AM1476; Biosyngen Announces FDA Fast Track Designation for BST02; Acepodia Announces FDA Clearance of IND Application for ACE2016
4DMT Presents Positive Interim Data from Randomized Phase II PRISM Clinical Trial of Intravitreal 4D-150 Demonstrating Favorable Tolerability & Clinical Activity in Wet AMD 4D Molecular Therapeutics, a prominent company in the field of genetic medicines with a focus on harnessing the full potential of geneti...
Read More...
Jan 30, 2024
Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR T Cell Therapy Abecma for Multiple Myeloma; FDA Approves Dupixent for Eosinophilic Esophagitis; Juvena Receives FDA Orphan Drug Designation for JUV-161; European Commission Authorizes GSK’s Omjjara; ENHERTU Granted Priority Review in the US for for metastatic HER2-positive Solid Tumors
Merck’s KEYTRUDA Reduced the Risk of Death by 38% Versus Placebo as Adjuvant Therapy for Patients With Renal Cell Cancer (RCC) at an Increased Risk of Recurrence Following Nephrectomy Merck, also known as MSD beyond the United States and Canada, has revealed findings from the Phase III KEYNOTE-564 trial, which a...
Read More...









-Agonist.png)

