Sanofi
Apr 30, 2019
Notizia
A Praluent boost for Sanofi, and Regeneron The U.S. FDA has given its nod to Praluent (alirocumab) to be used for reducing the risk of heart attack, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular (CV) disease. Praluent is an injectable prescription medicine co-dev...
Read More...
Jul 19, 2018
Business Cocktail
Sanofi bags rights to Revolution’s SHP2 cancer drug Sanofi has paid $50 million to work with Revolution Medicines on the development of cancer drugs against SHP2. The partners plan to move small molecule SHP2 inhibitor RMC-4630 into the clinic later this year. Sanofi has also committed to covering the R&D costs ...
Read More...
Jun 22, 2018
Fatty Acid Amide Hydrolase (FAAH) Inhibitor – an enzyme with novel therapeutic potential
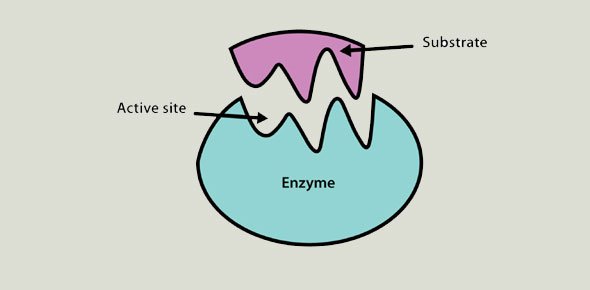
Fatty acid amide hydrolase (FAAH) belongs to the serine hydrolase family of enzymes. The identification of this enzyme was led by the discovery and characterization of fatty acid amides, including anandamide and oleamide, as a fundamental class of endogenous signaling molecules. FAAH serves as the major metabolic re...
Read More...
Mar 15, 2018
Business Cocktail
M&A activity of Sanofi and Bioverative for rare blood diseases Sanofi has strengthened its position in hematology & speciality medicine by acquiring Bioverativ, Biogen hemophilia & blood disorders-focused company for USD 105 per share on March 8, 2018. This will increase Sanofi’s hemophilia portfolio for...
Read More...
Mar 14, 2018
Cholesterol drug Praluent successful in reducing heart risk
Sanofi and Regeneron Pharmaceuticals got effective results in reducing the risk of major adverse heart events in a clinical study of nearly 19,000 patients with the help of cholesterol drug Praluent. Drugmakers now hope that the results are good enough to convince insurers to pay for the pricey medicine. Treatment w...
Read More...
Mar 13, 2018
Notizia
Anthera’s Sollpura flops after phase 3 Anthera cut free it’s flopped late-arrange Lilly castoff Sollpura (liprotamase), and now looks for those feared "key choices". Sollpura was out-licensed by Lilly in 2014, and the written work had all the attributes of its failure, back in December 2016 when it posted the info...
Read More...
Dec 19, 2017
Regeneron/Sanofi’s PD-1 drug candidate; Zika targeting drugs and vaccines developments; CMO of Achillion will leave this month; Lincoln’s anti-malarial drug
Regeneron/Sanofi’s PD-1 drug candidate will be tested with ISA’s HPV immunotherapy Regeneron and Sanofi’s PD-1 candidate cemiplimab is paired with Dutch biotech ISA Pharma’s human papillomavirus (HPV) targeting drug for testing. The new drug is competing to become the sixth PD-1/PD-L1 drug on the market. Joined with...
Read More...
Dec 14, 2017
Roche’s Phase 2 data; Celgene and Bluebird’s CAR-T therapy; Regeneron/Sanofi gets results; Allergan on women’s health
Positive Phase 2 data for Roche’s lymphoma ADC Roche’s antibody-drug conjugate for non-Hodgkin lymphoma polatuzumab has already received breakthrough designation from the USFDA and priority medicine status in the EU, and the expectations for the same are running pretty high. Phase 2 data presented by the company at...
Read More...
Jun 15, 2017
Sanofi invests; ICER pitches; $6M in NIH; Pfizer, Roche cancer drug pricing
Sanofi investing around $2B into biologics production over next 3 years Following the recent approval of two new biologics, Dupixent and Kevzara, and a pipeline stuffed full of large-molecule drugs, Sanofi will invest as much as €2 billion on its biologics manufacturing network over the next several years. The discl...
Read More...
Jun 13, 2017
Invokana matches; Xultophy beats; Lilly and BI follow; Tresiba matches
J&J's Invokana matches Jardiance's 14% reduction in major CV events There’s officially another heart-helping SGLT2 on the block. But this one brings some varied benefits—and amputation risks—along with it. In a cardiovascular outcomes trial, Johnson & Johnson's Invokana matched up to Eli Lilly and Boehringer...
Read More...






-Agonist.png)

