Sarcopenia
Mar 18, 2025
FDA Expands SOLIRIS for Pediatric Myasthenia Gravis; vTv’s Cadisegliatin Program Resumes as FDA Lifts Hold; ENCell’s EN001 Wins Orphan Drug Designation for Charcot-Marie-Tooth; FDA Accepts Sydnexis’ NDA for SYD-101 in Pediatric Myopia; Cambium Bio’s Potency Assay Strategy Cleared for Elate Ocular Phase III Trials
MDA Applauds FDA’s Expanded Approval of Soliris for Pediatric Generalized Myasthenia Gravis The Muscular Dystrophy Association (MDA) celebrates the FDA approval of an expanded indication for Alexion/AstraZeneca’s eculizumab (Soliris), now authorized for pediatric patients aged six and older with generalized myas...
Read More...
Jul 11, 2023
FDA Grants Priority Review for Zolbetuximab BLA; FDA Traditional Approval for LEQEMBI for Alzheimer’s Disease; Iovance Announces Regulatory and Clinical Updates for TIL Therapy in Advanced NSCLC; Biophytis Seeks FDA Approval to Launch Phase 3 Study of Potential Treatment of Sarcopenia; Orphan Drug Designation to Marker Therapeutics’s MT-401 for AML Treatment; Axsome Therapeutics Initiates Phase 3 Trial of Solriamfetol for ADHD
Astellas Announces FDA Grants Priority Review for Zolbetuximab Biologics License Application Astellas Pharma Inc. announced that the FDA has accepted and granted Priority Review for the company's Biologics Licence Application (BLA) for zolbetuximab, a first-in-class investigational Claudin 18.2 (CLDN18.2)-target...
Read More...
Mar 31, 2017
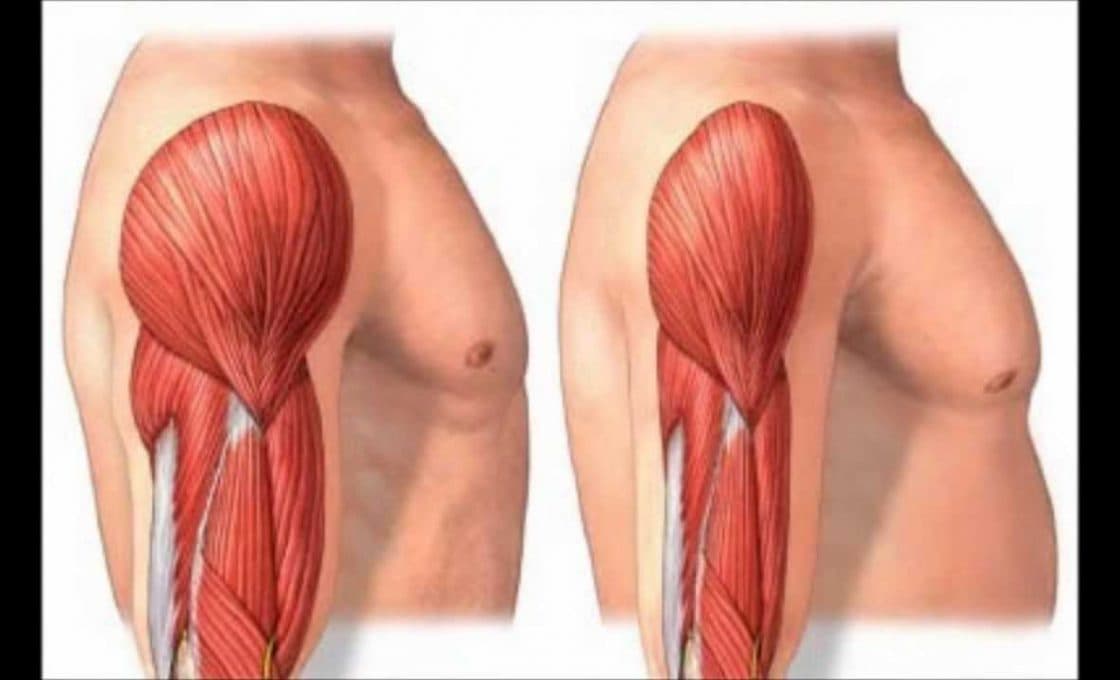
Sarcopenia: “Aging-Associated Strength Loss Condition”
Sarcopenia is an age-related geriatric syndrome which involves the loss of strength of skeletal muscles. The condition leads to impaired mobility, increased disability, more number of falls, as well as loss of physical function. Sarcopenia is observed in both physically inactive and active people, where factors such...
Read More...


-Agonist.png)

