Sepsis
Nov 20, 2024
Rising Acute Respiratory Distress Syndrome (ARDS) Prevalence Posing a Major Public Health Concern
The acute respiratory distress syndrome (ARDS) prevalence is increasing every year; it is estimated that in the United States alone, about 190K Americans are diagnosed with ARDS each year. Prior to the COVID-19 global pandemic, more than 700K individuals in the US and 2 million cases globally developed ARDS from tr...
Read More...
Aug 18, 2022
CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath Secures $10 Million Funding; BD, Accelerate Diagnostics Announce Collaboration; Avails Medical’s Clinical Trials for eQUANT; Movano Ring Exceeds Accuracy Targets for SpO2 & Heart Rate Monitoring
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System On August 09, 2022, CereVasc, Inc., a privately held, clinical-stage medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved ...
Read More...
Mar 03, 2022
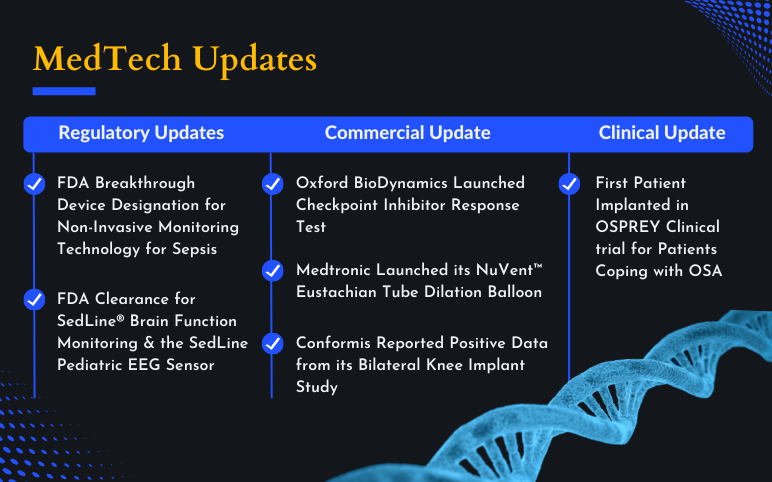
Noninvasix’s LIVOx™ Central Venous Oxygenation Monitor; LivaNova’s aura6000 System; Oxford BioDynamics’s EpiSwitch® CiRT; Masimo’s SedLine® Brain Function Monitoring and the SedLine Pediatric EEG Sensor; Medtronic’s NuVent™ Eustachian Tube Dilation Balloon; Conformis’s published data for Bilateral Knee Implant Study
Noninvasix Granted the US FDA Breakthrough Device Designation for Non-Invasive Monitoring Technology for Sepsis On February 23, 2022, Noninvasix, Inc. received the US FDA breakthrough device designation for its LIVOx™ Central Venous Oxygenation Monitor. It is a non-invasive device and provides real-time, ...
Read More...
Dec 02, 2021
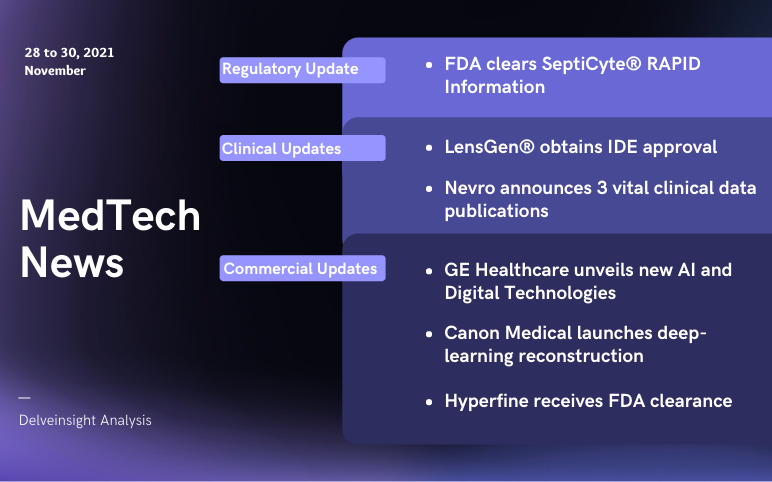
LensGen’s Juvene Intraocular Lens; Nevro announces clinical data publications; FDA Clearance to Immunexpress’s SeptiCyte; GE unveils AI and Digital Technologies; Canon launches PIQE DLR and SilverBeam; Hyperfine’s Swoop gets FDA clearance
Canon Medical launches super-resolution deep-learning reconstruction for Cardiac CT scans On November 28, 2021, Canon Medical launched the Precise IQ Engine (PIQE) DLR and SilverBeam filter in the recent version of the Aquilion ONE / PRISM edition. PIQE, super-resolution deep-learning reconstruction techno...
Read More...
Sep 21, 2021
Biogen’s AMD Biosimilar Gets Approval; SmartLabs Series B Funding; BARDA, Partner Therapeutics in Sepsis Space; Synlogic to Explore Sepsis Market Domain
Win-win for Biogen as its Biosimilar Gets Approval for AMD Samsung Bioepis and Biogen's biosimilar drug, Byooviz, received USFDA approval for the treatment of vision loss, Age-related macular degeneration and has become the first biosimilar to Lucentis in the AMD market. The Lucentis copycat had also manag...
Read More...



-Agonist.png)

