Sleep Apnea Diagnostic Devices
May 11, 2023
Norlase’s ECHO™ Green Pattern Laser; HAPPE Spine’sINTEGRATE-C™ Interbody Fusion System; Soterix Medical’s Parcel-Guided rTMS Depression Trial; Akura Medical’s High-Performance Mechanical Thrombectomy Platform; Intelivation Technologies’s Golden Isles™ Minimally Invasive Pedicle Screw System; Signifier Medical Technologies Announced Partnership with Sunrise
Norlase Received FDA 510(k) Clearance and CE Mark Approval For ECHO™ Green Pattern Laser On May 5, 2023, Norlase, a leading global ophthalmic laser manufacturer developing next-generation laser solutions, received both 510(k) US Food and Drug Administration clearance and CE Mark approval for the ECHO Gree...
Read More...
Feb 16, 2023
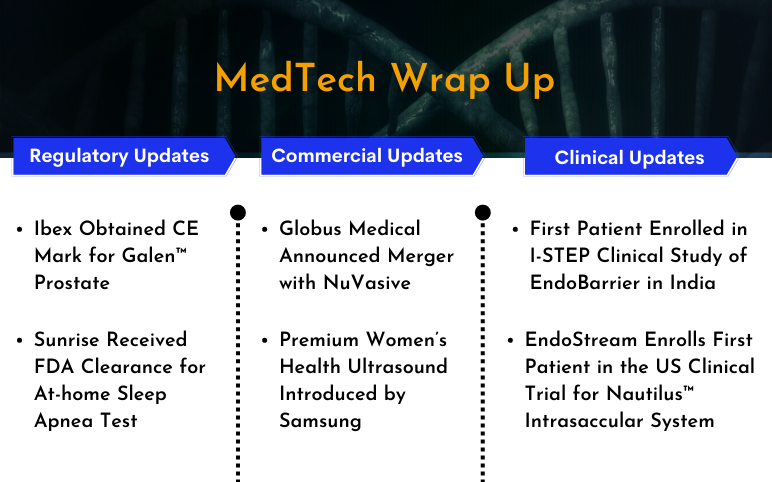
GI Dynamics’s I-STEP Clinical Study of EndoBarrier; EndoStream’s Clinical Trial for Nautilus™ Intrasaccular System; CE Mark for Ibex’s Galen™ Prostate; Sunrise’s At-home Sleep Apnea Test; Globus Medical Announced Merger with NuVasive; Boston Imaging’s HERA W10 Elite
First Patient Enrolled in I-STEP Clinical Study of EndoBarrier in India On February 13, 2023, GI Dynamics Inc., a medical device company, announced the enrollment of the first candidate in the I-STEP clinical trial in India. The trial aimed to assess the safety and effectiveness of the EndoBarrier® System for gl...
Read More...
Jan 12, 2023
Vivos Therapeutics ‘s DNA Oral Appliance for the Treatment of OSA; Arthrex’s TightRope Implant for Pediatric ACL Surgery; Chindex Medical Acquired 10 ViewRay MRIdian Systems; Zimmer Biomet to Acquire Embody; GE HealthCare to buy IMACTIS; Glaukos Announced the iDose TR Exchange Trial
Vivos Therapeutics Received FDA 510(k) Clearance for its Flagship DNA Oral Appliance for the Treatment of Obstructive Sleep Apnea On January 4, 2023, Vivos Therapeutics, Inc., a medical technology company focused on developing innovative treatments for patients suffering from dentofacial abnormalities and/or mil...
Read More...


-Agonist.png)

