Surgical Robotic Systems
Nov 20, 2025
Zimmer Biomet Secures FDA Clearance for Upgraded ROSA® Knee Robotic System; IceCure’s ProSense® Cryoablation Platform Secures Swissmedic Approval for Use in Breast, Lung, Liver, and Kidney Tumors; Clairity Secures $43M Series B to Launch FDA-Authorized AI Platform for Breast Cancer Risk Prediction; Distalmotion Secures $150M to Boost U.S. Rollout of DEXTER® Robotic Surgery System; Envoy Medical Reaches Six-Month Benchmark with First 10 Patients in Acclaim® Cochlear Implant Trial; Cardiovalve Seeks CE Approval After Positive TARGET Study Results
Zimmer Biomet Received U.S. FDA Clearance for Enhanced Version of ROSA® Knee Robotic Technology On November 14, 2025, Zimmer Biomet Holdings, Inc., a leading global medical technology company, announced that its enhanced ROSA® Knee with OptimiZe™ system received 510(k) clearance from the U.S. Food and Drug...
Read More...
Jun 23, 2025
How Robots Are Introducing A New Dimension To Healthcare Service Delivery
Artificial intelligence and robotics are two technologies that have demonstrated the potential to address and provide solutions to numerous contemporary issues. The manufacturing sector has been using robotics for quite a long time. However, over the past three to four decades, robots have been in use in other sect...
Read More...
May 01, 2025
Medtronic Receives FDA Approval for OmniaSecure™ Lumenless Defibrillation Lead and Presents Positive Clinical Trial Results for Hugo™ Robotic-Assisted Surgery System; Roche’s AI Companion Diagnostic for NSCLC Earns FDA Breakthrough Device Designation; Boston Scientific Announces Positive 12-Month Phase 2 Results from ADVANTAGE AF Trial of FARAPULSE™ PFA System; Bausch Health Launches Fraxel FTX™ to Advance Skin Rejuvenation Treatments; Gestalt Diagnostics Secures $7.5M in Series A to Scale AI Pathology Solutions
Medtronic Received FDA Approval for Smallest-Diameter, Lumenless Defibrillation Lead, the Omniasecure™ Lead and Published Investigational Clinical Study Results, Offering New Therapy Option for Heart Rhythm Disorders On April 25, 2025, Medtronic plc., a global leader in healthcare technology, received U.S....
Read More...
Mar 27, 2025
Alcon Gains CE Mark for Clareon Vivity IOL in Europe; MicroPort MedBot’s Toumai SP Robot Wins NMPA Approval; Abbott Launches Intravascular Lithotripsy Trial; BD Advances GalaFLEX LITE™ Clinical Trial; GE HealthCare Unveils AI-Powered Invenia ABUS Premium; Okami Medical Introduces SENDERO® MAX Catheter
Alcon Announced CE Mark Approval and Launch of Clareon Vivity IOL in Europe, Expanding Visual Options On March 25, 2025, Alcon, the global leader in eye care committed to enhancing vision, announced that Vivity®, the most widely implanted extended depth of focus (EDOF) intraocular lens (IOL), became availa...
Read More...
Nov 14, 2024
FDA Grants Approval to Caris Life Sciences for MI Cancer Seek; Johnson & Johnson MedTech Secures FDA IDE Approval for OTTAVA; Organogenesis Shares Positive Interim Data from Second Phase 3 Study of ReNu; LivaNova’s OSPREY Trial Achieves Key Safety and Efficacy Milestones; Hyperfine Launches Advanced Portable MR Brain Imaging Swoop® System Across Europe; Nihon Kohden Strengthens Neurological Solutions Through Ad-Tech Medical Instrument Corporation Acquisition
Caris Life Sciences Received FDA Approval for MI Cancer Seek™ as a Companion Diagnostic (CDx) Test On November 6, 2024, Caris Life Sciences, a leading next-generation AI TechBio company and precision medicine pioneer, announced that the U.S. Food and Drug Administration (FDA) approved MI Cancer Seek™ for u...
Read More...
Oct 31, 2024
Medtronic Secures FDA Green Light for Affera™ Mapping and Ablation System Alongside Sphere-9™ Catheter; Precision Optics Gets FDA 510(k) Clearance; Abbott Launches New Clinical Trial Aimed at Enhancing Care for Advanced Heart Failure Patients; Fresenius Medical Care’s Study Confirms Efficacy of New Anemia Therapy Software in Enhancing Outcomes for Hemodialysis Patients; Inspira™ Announces New Distribution Center to Support INSPIRA™ ART100’s U.S. Introduction; WellSky Expands Home Care Offerings with Acquisition of Bonafide
Medtronic Received FDA Approval for Affera™ Mapping and Ablation System and Sphere-9™ Catheter, Pioneering Advances in Arrhythmia Treatment On October 24, 2024, Medtronic plc, a global leader in healthcare technology, announced the United States Food and Drug Administration (FDA) approval of its Affera™ Ma...
Read More...
Sep 19, 2024
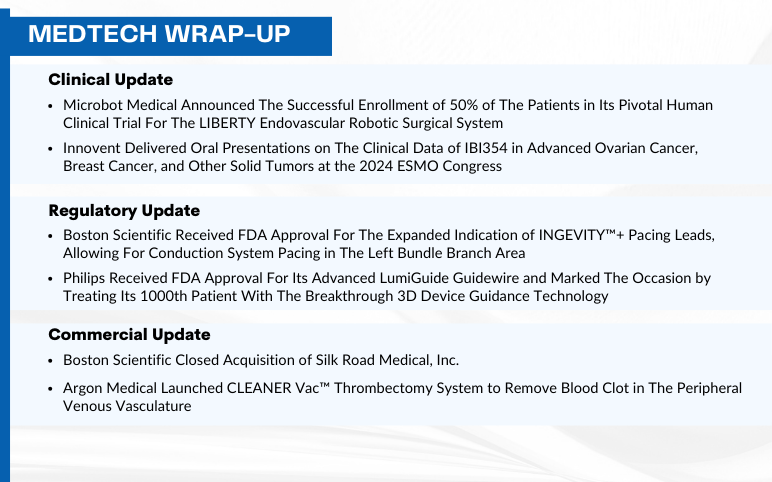
Boston Scientific INGEVITY™ gets Expanded Indication and acquires Silk Road Medical; FDA Approves Philips’ LumiGuide Guidewire; Microbot Completes LIBERTY Trial Enrollment; Innovent Presents Data at ESMO 2024; Argon Medical Launches CLEANER Vac™ System
Boston Scientific Received FDA Approval For The Expanded Indication of INGEVITY™+ Pacing Leads, Allowing For Conduction System Pacing in The Left Bundle Branch Area On September 17, 2024, Boston Scientific Corporation received U.S. Food and Drug Administration (FDA) approval to expand the indication for th...
Read More...
Aug 22, 2024
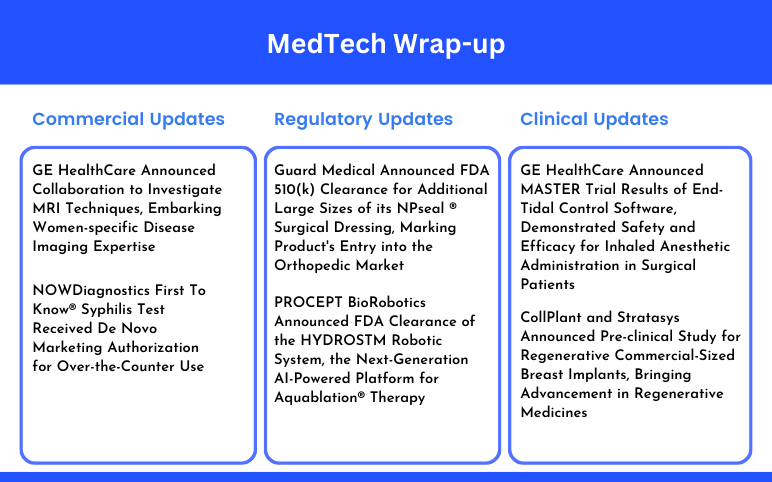
Guard Medical Announced FDA 510(k) Clearance; PROCEPT BioRobotics Announced FDA Clearance; GE HealthCare Announced MASTER Trial Results; CollPlant and Stratasys Announced Pre-clinical Study; GE HealthCare Announced Collaboration to Investigate MRI Techniques; NOWDiagnostics’ Syphilis Test Received De Novo Marketing Authorization
Guard Medical Announced FDA 510(k) Clearance for Additional Large Sizes of its NPseal ® Surgical Dressing, Marking Product's Entry into the Orthopedic Market On August 19, 2024, Guard Medical, Inc., a medical technology company, announced FDA 510(k) clearance for expanded large sizes (up to 25cm) of its advanced...
Read More...
Jun 13, 2024
Asensus Surgical Acquired by Karl Storz; Allosource® Launched Aceconnex®; Siemens Healthineers FDA Clearance for Chiller-Free Biograph Trinion PET/CT; Abbott Received FDA Clearance; Foldax® Reported Positive Clinical Results; Amber Implants Completed Enrolment of First-In-Human Clinical Trial
Asensus Surgical Agreed to be Acquired by Karl Storz On June 07, 2024, Asensus Surgical announced that it had entered a deal in which Karl Storz would acquire the company for 35¢ per share in cash. Karl Storz Endoscopy-America, a wholly-owned subsidiary of Germany-based Karl Storz, had agreed to acquire...
Read More...
Jun 06, 2024
Stryker’s LIFEPAK 35 Monitor/Defibrillator; Qiagen’s QIAstat-Dx Respiratory Panel; Evolution Optiks’s 510(k) Clearance; Microbot Medical Endovascular Surgical Robot Trial; Nexalin’s Insomnia and Anxiety Therapy Headset Clinical Trial; KORU Medical Systems’ Study With Commercialized Oncology Biologics
Stryker Released LIFEPAK 35 Monitor/Defibrillator On June 04, 2024, Stryker, a global leader in medical technologies, announced the launch of the LIFEPAK 35 monitor/defibrillator. This latest addition to their monitor/defibrillator lineup features advanced technology and is designed on an intuitive, modern...
Read More...







-Agonist.png)

