Total Artificial Heart
Aug 01, 2024
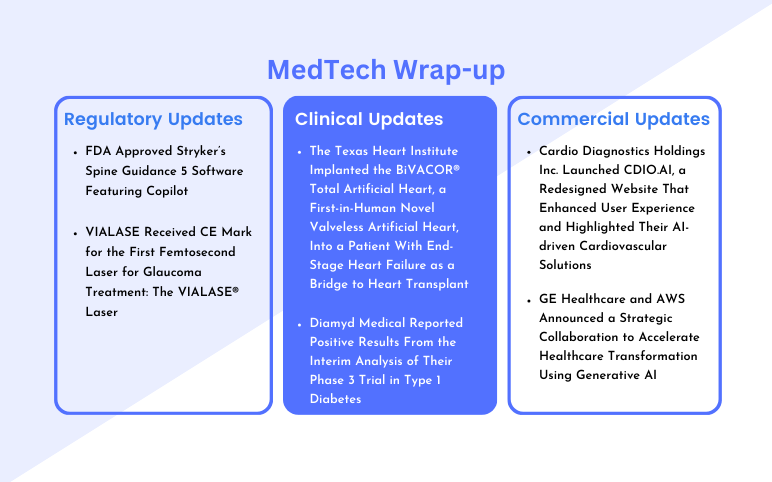
Stryker’s Spine Guidance 5 Software FDA Approval; VIALASE Received CE Mark; The Texas Heart Institute Implanted the BiVACOR® Total Artificial Heart; Diamyd Medical’s Phase 3 Trial Positive Results; Cardio Diagnostics Holdings Inc.’s CDIO.AI; GE Healthcare and AWS Strategic Collaboration
FDA Approved Stryker’s Spine Guidance 5 Software Featuring Copilot On July 30, 2024, Stryker, a global leader in medical technologies, announced that its Q Guidance System with Spine Guidance 5 Software featuring Copilot received 510(k) clearance from the U.S. Food and Drug Administration. This first-to-market t...
Read More...
Dec 07, 2023
Johnson & Johnson Medtech Acquired Laminar; BD Launched Advanced Vascular Access Ultrasound System; FDA Cleared Oral Device for Severe Sleep Apnea; FDA Clearanced the Zilia OcularTM FC Retinal Camera; First Patient Enrolled in Penumbra Study of Computer-assisted Vacuum Thrombectomy; US FDA Granted the BiVACOR Total Artificial Heart IDE Approval for First-in-Human Early Feasibility Study
FDA Cleared Oral Device for Severe Sleep Apnea From Vivos Therapeutics On November 29, 2023, Vivos Therapeutics announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Vivos’s removable CARE (Complete Airway Repositioning and Expansion) oral appliances developed for treat...
Read More...

-Agonist.png)

