Transcatheter Aortic Valve Replacement Devices
Dec 26, 2024
FDA Grants Approval to Merit Medical’s WRAPSODY Endoprosthesis; FDA Clears AVITA Medical’s Cohealyx, Expanding Treatment Potential; SeaStar Medical Launches 14th Location for Adult AKI Pivotal Study; Contego Medical’s Neuroguard IEP System Treats First Patients; SMT Introduces Hydra™ TAVI System in Russia; SandboxAQ Raises $300M+ to Accelerate AI’s Next Frontier
Merit Medical Announced FDA Approval of the WRAPSODY Cell-Impermeable Endoprosthesis On December 20, 2024, Merit Medical Systems, Inc., a leading global manufacturer and marketer of advanced healthcare technologies, announced that its WRAPSODY® Cell-Impermeable Endoprosthesis received premarket approval (P...
Read More...
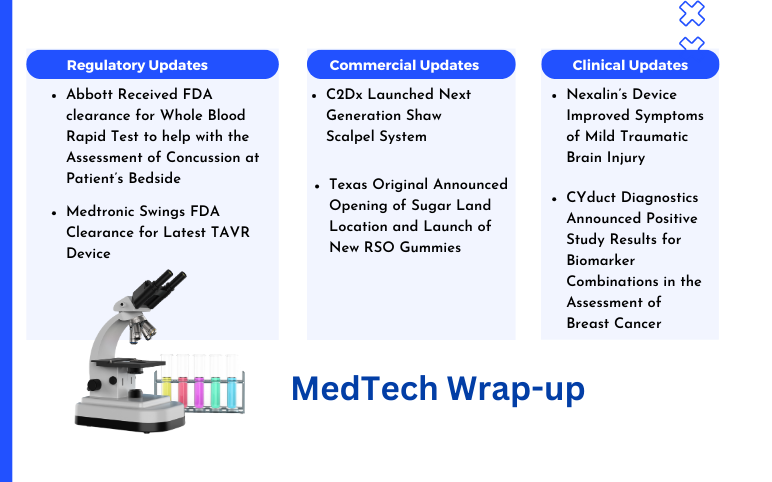
Apr 04, 2024
C2Dx’s Shaw Scalpel System; Texas Original’s RSO Gummies; Abbott’s Whole Blood Rapid Test FDA Clearance; Medtronic’s TAVR Device FDA Clearance; Nexalin’s Device Improved Symptoms; CYduct Diagnostics’ Positive Study Results
C2Dx Launched Next Generation Shaw Scalpel System On March 26, 2024, C2Dx, a privately-owned medical device company, announced the launch of its Next Generation Shaw Scalpel System. The Shaw Scalpel System has obtained 510(k) clearance from the FDA for the upgraded controller of its advanced surgical techn...
Read More...
Jun 16, 2022
Aurora Spine’s Interbody Fusion Device; LENSAR’s ALLY Adaptive Cataract Treatment System; Abbott’s Mitral & Tricuspid Heart Valve Devices; Boston Scientific’s Acurate Neo2 Aortic Valve System Study; Resolution Medical Acquired Lifetec Group; Paragon 28’s Monkey Rings External Fixation System
US FDA Grants 510K Clearance for Aurora Spine’s Interbody Fusion Device On June 07, 2022, Aurora Spine, Inc. a designer and manufacturer of innovative medical devices, is an emerging growth company focused on delivering new solutions to the spinal implant and pain management industries through a series of screwl...
Read More...
May 05, 2022
GE and Medtronic’s Collaboration; Vivalink’s Multi-Vital Blood Pressure Patch; Foldax’s TRIA Biopolymer Surgical Aortic Heart Valve; Vektor Medical’s vMap Clinical Validation Study; iSono’s ATUS System; Mirvie’s Test to Identify Risk of Preeclampsia
GE Healthcare and Medtronic Collaborated to Meet the Increasing Need for Outpatient Care On April 28, 2022, GE Healthcare and Medtronic entered into a collaboration to provide patients, clinicians, and payers seeking more choices of excellent care, inside and outside of the traditional hospital without compromis...
Read More...
Dec 23, 2021
Sky Medical’s Geko™ device; Fist Assist Devices obtains Breakthrough Device designation; Edwards obtains FDA approval; BD launched instrument for testing; GE acquires BK Medical; Zynex’ CM-1500; Foldax’s TRIA biopolymer heart valve
Sky receives FDA clearance to market the geko™ device for venous insufficiency and ischemia On December 16, 2021, Sky Medical Technology Ltd, a medical device company, got approval from the US Food and Drug Administration (FDA) 510(k) to market the geko™ device for growing microcirculatory bl...
Read More...




-Agonist.png)

