Ultrasound Devices
Mar 20, 2025
Illuccix Receives Dutch Approval for PSMA-PET Imaging in Prostate Cancer; FDA Approves Valcare Medical’s Early Feasibility Study for Novel Amend™ Trans-Septal System; RevBio Launches Pivotal Clinical Trial in Europe for Dental Implant Stabilization; Insulet’s RADIANT Trial Highlights Improved Glycemic Outcomes with Omnipod® 5 Following Direct Transition; Envoy Medical Raises $10 Million to Drive Clinical Progress on Next-Gen Hearing Device; Vave Health Unveils World’s First Wireless Whole-Body Ultrasound with Single PZT Transducer
Illuccix® Prostate Cancer PSMA-PET Imaging Agent Approved in the Netherlands On March 18, 2025, Telix announced that its prostate cancer PET imaging agent, Illuccix® (kit for the preparation of gallium-68 gozetotide injection), received marketing authorization from the Medicines Evaluation Board (MEB) in t...
Read More...
Sep 26, 2024
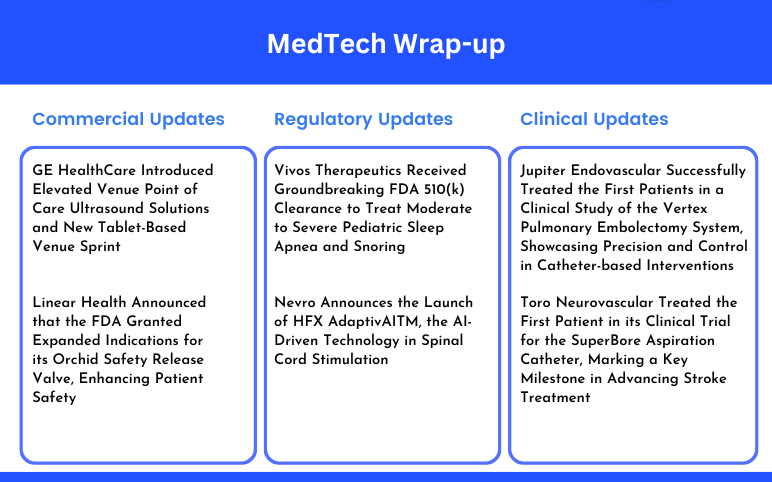
Vivos Therapeutics Receives FDA 510(k) Clearance for Pediatric Sleep Apnea; Nevro Launches AI-Driven Spinal Cord Stimulation; Jupiter Endovascular Treated First Patients in Pulmonary Embolectomy System Study; Toro Neurovascular Advances Stroke Treatment with SuperBore Aspiration Catheter; GE HealthCare Introduces Venue Ultrasound Solutions; Linear Health Expands Orchid Safety Valve Indications
Vivos Therapeutics Received Groundbreaking FDA 510(k) Clearance to Treat Moderate to Severe Pediatric Sleep Apnea and Snoring On September 18, 2024, Vivos Therapeutics, Inc., a premier medical device and technology firm focused on innovative treatments for sleep-related breathing disorders (SRBDs), including obs...
Read More...
Aug 29, 2024
Insulet Corporation’s Omnipod® 5 Automated Insulin Delivery System Receives FDA Approval; FDA Gives Green Light to Expand Vericel’s MACI Arthro Label; SkinCure Oncology Announces New Study of Non-melanoma Skin Cancer Through Image-guided Superficial Radiation Therapy; SDC’s BYOD ePRO Solution Clinical Trial; PaceMate® Acquires Paceart Optima™ System; KARL STORZ Acquires a US-headquartered Company
FDA Cleared the Omnipod® 5 Automated Insulin Delivery System for Use in People with Type 2 Diabetes On August 26, 2024, Insulet Corporation, the global leader in tubeless insulin pump technology with its Omnipod® brand of products, announced that its groundbreaking Omnipod 5 Automated Insulin Delivery Syst...
Read More...
Jul 25, 2024
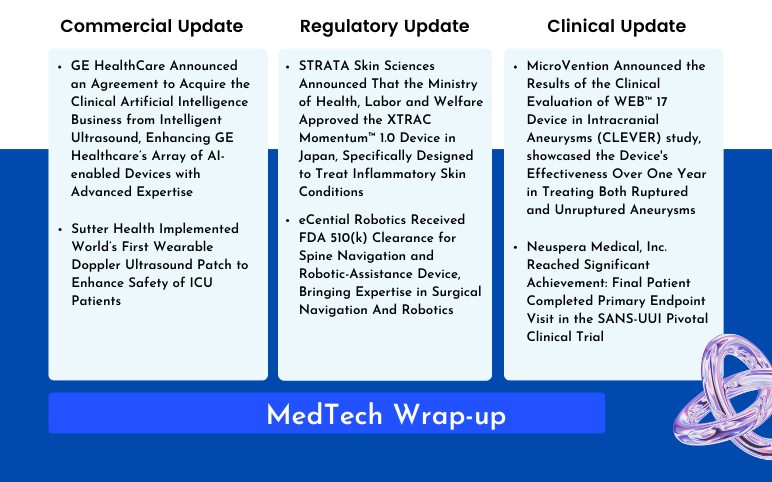
STRATA Skin Sciences’ XTRAC Momentum™ 1.0 Device Approved in Japan; eCential Robotics Received FDA 510(k) Clearance for Spine Navigation and Robotic-Assistance Device; MicroVention Announced the Results of the Clinical Evaluation of WEB™ 17 Device; Neuspera Medical, Inc.’s SANS-UUI Pivotal Clinical Trial; GE HealthCare Announced an Agreement to Acquire the Clinical Artificial Intelligence Business; Sutter Health Implemented World’s First Wearable Doppler Ultrasound Patch
STRATA Skin Sciences Announced That the Ministry of Health, Labor and Welfare Approved the XTRAC Momentum™ 1.0 Device in Japan, Specifically Designed to Treat Inflammatory Skin Conditions On July 22, 2024, STRATA Skin Sciences, Inc., a medical technology company specializing in developing, commercializing, and m...
Read More...
May 16, 2024
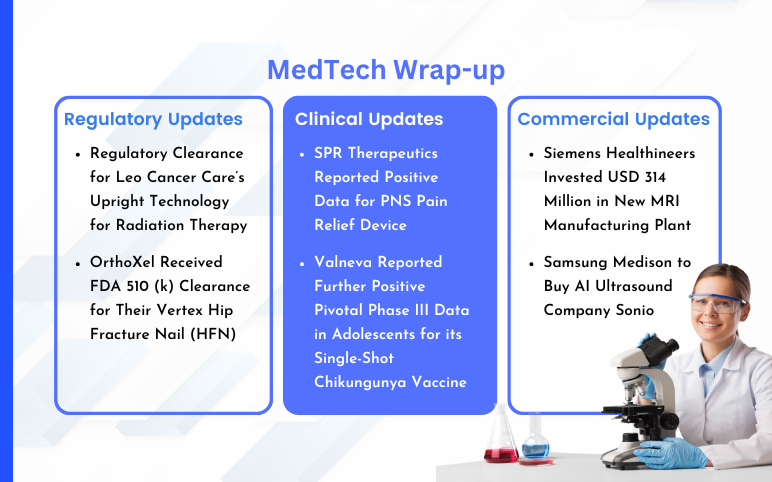
Siemens Healthineers USD 314 Million Investment; Samsung Medison’s Sonio Acquisition; Leo Cancer Care’s Upright Technology Clearance; OrthoXel’s Vertex Hip Fracture Nail FDA 510 (k) Clearance; SPR Therapeutics’ PNS Pain Relief Device Positive Data; Valneva’s Chikungunya Vaccine Positive Pivotal Phase III Data
Siemens Healthineers Invested USD 314 Million in New MRI Manufacturing Plant On May 15, 2024, Siemens Healthineers announced that it had invested USD 314 million in a new MRI manufacturing plant in the UK. The facility in Oxford, England, which Siemens anticipates opening in 2026, will develop technology to redu...
Read More...
Apr 13, 2023
BD’s Advanced Ultrasound Technology for IV Insertions; Compal Electronics Launched New RFA System AblatePal; Icentia Received Clearance for CardioSTAT; Avation Medical Announced Clearance for the Vivally® System; Cardiovascular Systems’s ECLIPSE Clinical Trial; Bone Biologics Received Approval to for Pilot Trial with NB1 in Spinal Fusion Patients
BD Introduced Advanced Ultrasound Technology for IV Insertions to Help Drive First-Stick Success On April 11, 2023, BD (Becton, Dickinson, and Company), a leading global medical technology company, announced the launch of a new, easy-to-use advanced ultrasound device with a specialized probe designed to provide ...
Read More...
Mar 30, 2023
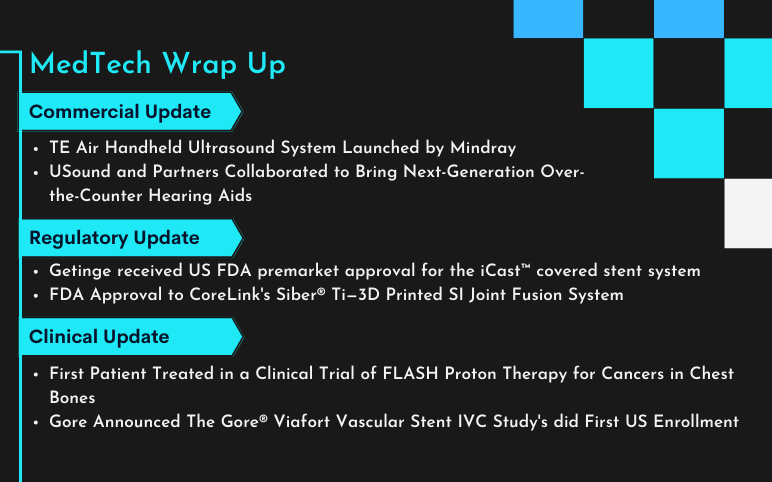
Mindray’s TE Air Handheld Ultrasound System; Getinge’s iCast™ Covered Stent System; Clinical Trial Update for the FLASH Proton Therapy for Cancers in the Bones; Gore’s Gore® Viafort Vascular Stent IVC Study; CoreLink’s Siber® Ti—3D Printed SI Joint Fusion System; USound and Partners’s Next-Generation Over-the-Counter Hearing Aids
TE Air Handheld Ultrasound System Launched by Mindray On March 16, 2023, Mindray, a China-based company announced the launch of TE Air, a high-quality, wireless, handheld, portable ultrasound device that is designed to easily fit into a pocket. Built with Mindray’s eWave platform and second-generation S...
Read More...



-Agonist.png)

