Vertex Pharmaceuticals
Jan 12, 2024
Is Bacteriophage a Potential Game-Changer in the Realm of Cystic Fibrosis Treatment?
Cystic Fibrosis impacts approximately 1 in every 3,500 births, with around 70,000 affected individuals globally, and 31,000 in the United States alone. Those with the condition experience the development of dense, adhesive mucus in their lungs, making them prone to infections. Over time, this leads to inflammation ...
Read More...
Dec 15, 2023
Lyfgenia or Casgevy: Who Will Lead the Sickle Cell Disease Treatment Space?
History has been created as the world captures the significance of the FDA’s approval of Casgevy (exa-cel), a collaboration between Vertex Pharmaceuticals and CRISPR Therapeutics, for the treatment of sickle cell disease (SCD). This groundbreaking therapy represents a long-awaited potential cure for the debilitatin...
Read More...
May 01, 2023
Cell and Gene Therapies for Diabetes Treatment: A Permanent Cure for Patients?
Diabetes is the 8th largest cause of death in the United States (although its prevalence may be underreported). Diabetes affects more than 37 million people in the United States, and 1 in every 5 are unaware of their condition. Over 96 million US adults—more than one-third—have prediabetes, and more than 8 out of 1...
Read More...
Apr 14, 2023
Vertex/CRISPR’s Gene-editing Therapy exa-cel: Inch Ahead of Rival
Vertex Pharma and CRISPR Therapeutics are the first companies to seek FDA clearance for a gene-editing therapy. Vertex Pharmaceuticals and CRISPR Therapeutics have gotten closer to introducing exagamglogene autotemcel (exa-cel), a one-time treatment for sickle cell disease (SCD) and transfusion-dependent beta-...
Read More...
Apr 11, 2023
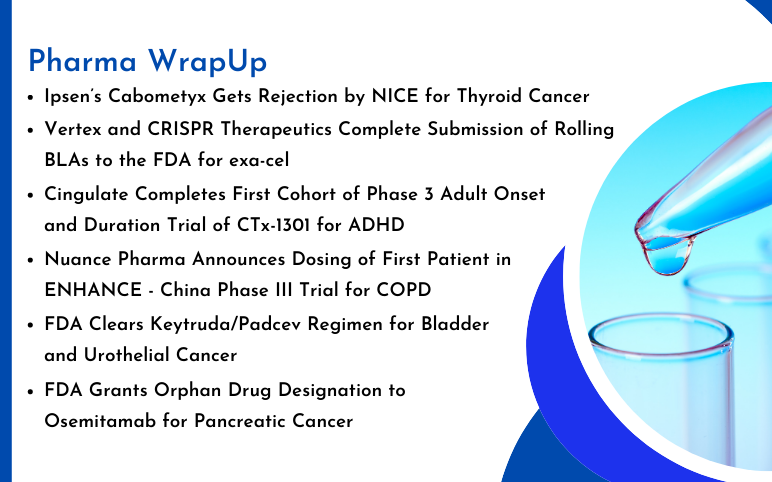
Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for exa-cel; Orphan Drug Designation to Osemitamab for Pancreatic Cancer; FDA Clears Keytruda/Padcev for Bladder and Urothelial Cancer; Cingulate Completes Trial of CTx-1301 for ADHD; Nuance Pharma Announces Dosing of First Patient in ENHANCE Trial
FDA Grants Orphan Drug Designation to Osemitamab for Pancreatic Cancer Transcenta Holding Limited has announced that the U.S. Food and Drug Administration (FDA) has awarded Orphan Drug Designation to Osemitamab (TST001), a highly potent humanized monoclonal antibody that enhances ADCC (antibody-dependent cell-me...
Read More...
Aug 21, 2018
Kalydeco got approved; Opdivo for SCLC; FDA approved for pain relief; GC4419 obviates
Kalydeco, the first Cystic Fibrosis drug of Vertex, got approved for infants The Food and Drug Administration has cleared Kalydeco drug of Vertex Pharmaceuticals for infants aged 12 to under 24 months. The approved drug treats the underlying cause of cystic fibrosis in children of this age with at least one mutatio...
Read More...



-Agonist.png)

