X-Ray Devices Market
Aug 08, 2024
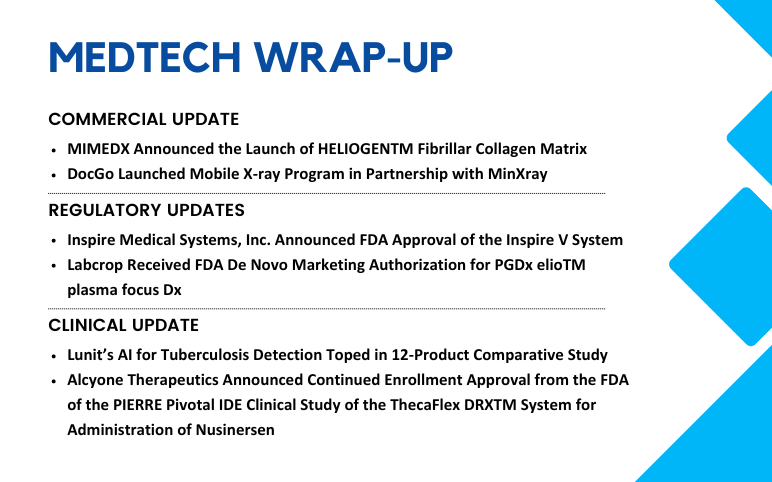
MIMEDX Launches HELIOGENTM Fibrillar Collagen Matrix; DocGo Launches Mobile X-ray Program; Labcrop’s FDA De Novo Marketing Authorization; Inspire Medical Systems Receives FDA Approval; Alcyone Therapeutics Announces Continued Enrollment Approval from the FDA; Lunit’s AI for Tuberculosis Detection
MIMEDX Announced the Launch of HELIOGENTM Fibrillar Collagen Matrix On July 31, 2024, MiMedx Group, Inc. launched HELIOGEN™ Fibrillar Collagen Matrix, a particulate xenograft product designed to treat complex wounds, especially in surgical environments. HELIOGEN is a shelf-stable product that contains Type...
Read More...
Aug 11, 2022
Rapid Medical’s TIGERTRIEVER 13; Glaukos’s Istent Infinite System; GE Healthcare’s Definium 656 HD; NeuroOne’s Signed Exclusive Development & Distribution Agreement with Zimmer; Kaia Health’s Rise-uP Randomized Controlled Trial; Bluejay’s Symphony IL-6 Test
Rapid Medical Obtains FDA Clearance for the World's Smallest and Only Adjustable Thrombectomy Device On July 26, 2022, Rapid Medical, a leading developer of advanced neurovascular devices, received Food and Drug Administration (FDA) 510(k) clearance for TIGERTRIEVER™13 for large vessel occlusions.&n...
Read More...
Apr 07, 2022
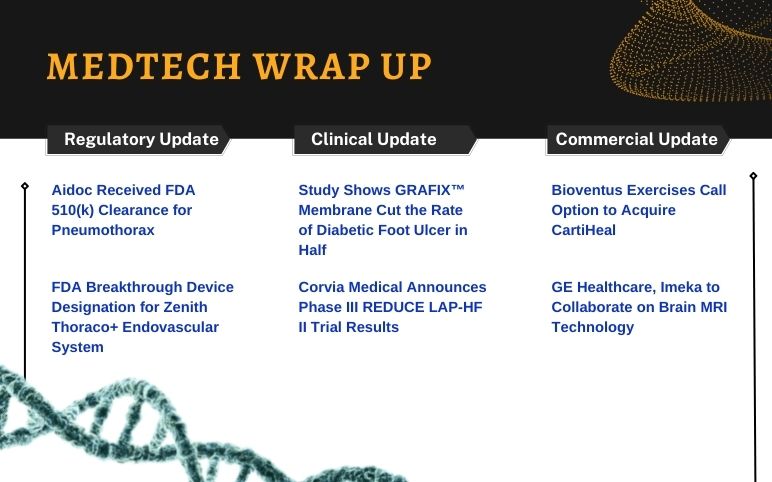
Aidoc’s Pneumothorax; Cook Medical’s Zenith Thoraco+ Endovascular System; GRAFIX Membrane Study; Corvia’s REDUCE LAP-HF II Trial Results; GE Healthcare and Imeka’s Collaboration; Bioventus to Acquire CartiHeal
Aidoc Expands AI Service to X-ray, Receiving FDA 510(k) Clearance for Pneumothorax On March 30, 2022, Aidoc, the leading developer of healthcare AI solutions, announced that its triage and notification of pneumothorax on X-ray exams has gained FDA 510(k) approval. Aidoc's other seven FDA-cleared clinical AI prod...
Read More...

-Agonist.png)

