Yescarta
Sep 02, 2024
CAR T-Cell Therapies in Non-Hodgkin’s Lymphoma Treatment: A Revolutionary Approach
CAR-T cells offer a lasting benefit through a single treatment, sparing patients with high-risk conditions from the toxicity associated with salvage chemotherapy and autologous transplants. These approvals have changed the standard of care for patients who are either resistant to initial treatment or experience ear...
Read More...
Dec 16, 2022
ASH 2022: Yescarta emerging as the CAR-T leader in DLBCL; Failure of Kymriah in early lines of DLBCL
CAR T-cell therapy is a type of immunotherapy that uses your body's white blood cells to fight your lymphoma. It uses a particular kind of white blood cell known as T lymphocytes or T cells. An essential part of CART therapies is lymphodepleting conditioning, which is administered to the patient before infusion of ...
Read More...
Apr 02, 2021
A New Addition to Existing Chimeric Antigen Receptor Therapies –Potential for a Game Changer in Blood Cancers
Chimeric Antigen Receptor (CAR) Therapies have changed the treatment paradigm of hematological malignancies to a vast extent. These personalized therapies have not only evolved as a game-changer in the field of medicine but also in the lives of the patients. Until the last approvals made by Novartis and Kite Pha...
Read More...
May 28, 2020
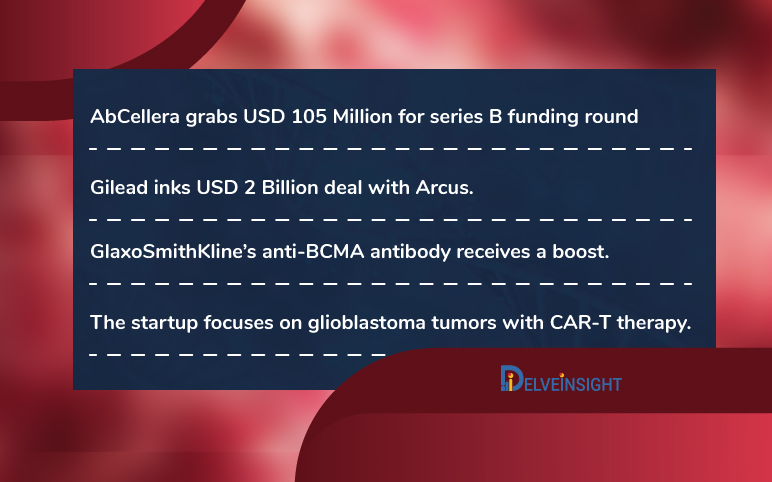
Startup focuses tumors with CAR-T; AbCellera grabs USD 105M; Gilead inks USD 2B deal; GSK’s anti-BCMA antibody receives a boost
The startup focuses on glioblastoma tumors with CAR-T therapy. CAR-T technology that involves genetically modifying a patient’s immune cells to recognize and attack cancer. However, while the innovation has helped patients with certain blood malignancies, progress in solid tumors remains restricted. The scien...
Read More...
Feb 18, 2020
Why should every company working on CAR-T therapy attend CAR TCR Summit Europe?
The third edition of CAR-TCR Summit Europe is a one-stop platform that provides attendees with a holistic view of the Gene therapy market, listing the solutions to the plausible hurdles that come across in the process. Hosting over 1000 industry-experts, the CAR TCR Summit is tailored for pharma & biotech play...
Read More...
Aug 31, 2018
Commercial
NICE Rejects Gilead Sciences’ Yescarta on Cost Concerns The United Kingdom’s government-funded health service isn’t being so nice to Gilead Sciences’ CAR-T blood cancer treatment Yescarta. The country’s National Institute for Health and Care Excellence (NICE) said the therapy is too expensive for th...
Read More...
Aug 29, 2018
Snippet
National health authorities, U.K.'s NICE determines Gilead's cancer cell therapy exorbitant The National Institute for Health and Care Excellence (NICE) found Gilead's cancer cell therapy, Yescarta to be clinically efficacious for treating a type of lymphoma, but no ample evidence is present to estimate the benefit...
Read More...
Feb 14, 2018
Gilead Sciences Introduces Sovaldi: A Potent Hepatitis C Treatment
Gilead Sciences has recently launched a highly effective hepatitis C drug Sovaldi at a whopping price of USD 84,000 per treatment course at the same time when ‘How to price a cure’ discussion has entered the pharma industry. Gilead bought a launch-ready drug and launched it very quietly hoping that it's very cost-ef...
Read More...




-Agonist.png)

