The Rise of TCR Therapy: A Beacon of Hope in Cancer Treatment
Jan 01, 2024
Table of Contents
T-cell receptor (TCR)-dependent treatment involves modified immune cells aimed at attacking particular tumor indicators. This treatment approach necessitates a systematic procedure including patient evaluation (e.g., for HLA-A*02:01 and precise tumor markers), collecting immune cells, creating the altered TCR product, reducing immune activity, and administering the TCR-based treatment.
The Role of TCRs in Recognizing and Targeting Abnormal Cells
TCRs play a pivotal role in the immune system’s ability to recognize and target abnormal cells within the body. These specialized proteins are expressed on the surface of T cells, a type of white blood cell that orchestrates the immune response. The primary function of TCRs is to interact with antigenic peptides presented on the surface of cells, distinguishing between normal, healthy cells and those that may be infected, cancerous, or otherwise abnormal.
Downloads
Article in PDF
Recent Articles
- FDA Grants Priority Review to BMS’ Luspatercept; Teva and MedinCell’s Risperidone FDA Approval; B...
- How is Nanomedicine Transforming the Dynamics of the Healthcare Industry?
- ESMO Asia 2022: Role of EGFR and ALK mutations in the East Asian Lung Cancer Market
- Cytotoxic T lymphocyte-associated antigen-4 Market Forecast & Insights
- Breaking Ground: Ibrutinib and Venetoclax Synergy Delivers Significant Progression-Free Survival ...
When cells undergo abnormal changes, such as mutations leading to cancer or viral infection, they often produce abnormal or foreign proteins. TCRs are designed to recognize these specific antigens, enabling T cells to identify and eliminate the aberrant cells. This recognition process is highly specific, as each TCR is tailored to recognize a particular antigenic peptide. This specificity is crucial for the immune system to target only the cells that pose a threat while preserving the integrity of healthy tissues.
Upon binding to the abnormal cell’s antigen, TCRs trigger a series of signaling events within the T cell, initiating an immune response. This response may involve the activation of other immune cells, such as cytotoxic T cells that directly attack and destroy the abnormal cells. Additionally, TCR engagement can stimulate the release of cytokines, which further regulate and amplify the immune response. The role of TCRs in immune surveillance and targeting abnormal cells underscores their significance in maintaining the body’s overall health and defending against diseases like cancer and infections. Understanding the intricacies of TCR recognition is essential for developing novel immunotherapies and therapeutic interventions to enhance the immune system’s ability to combat a wide range of disorders.
TCR Therapy vs. Other Immunotherapeutic Approaches: Advantages and Unique Features
TCR therapy stands out among various immunotherapeutic approaches due to its unique mechanism and distinct advantages. Unlike other immunotherapies that primarily rely on antibodies, such as monoclonal antibodies or checkpoint inhibitors, TCR therapy harnesses the power of T cells, a key component of the adaptive immune system. T cells are designed to recognize specific antigens, enabling them to target and eliminate infected or cancerous cells with high precision.
One of the key advantages of TCR therapy lies in its ability to target intracellular antigens, including those expressed by cancer cells. This is particularly crucial in the context of solid tumors, where traditional antibody-based approaches may face challenges penetrating the tumor microenvironment. TCR therapy allows for the precise identification and targeting of cancer cells based on specific peptide antigens presented on their surfaces, enhancing the therapy’s effectiveness against a broader range of malignancies.
Furthermore, TCR therapy offers a personalized treatment approach. It involves isolating T cells from a patient, genetically modifying them to express specific TCRs that recognize the target antigens, and then infusing these engineered T cells back into the patient. This personalized aspect allows for the customization of the treatment to each individual’s unique cancer profile, potentially increasing its efficacy.
Despite its promise, TCR therapy also faces challenges, such as the identification of suitable target antigens and potential off-target effects. Moreover, the complexity of the manufacturing process and the associated costs are areas that researchers and clinicians continue to address. Nonetheless, the unique features and advantages of TCR therapy make it a promising avenue in the rapidly evolving field of cancer immunotherapy, offering new hope for patients with challenging and resistant forms of cancer.
TCR Therapy in Action: Success Stories and Milestones
Immunotherapies have rapidly emerged as a cornerstone in the realm of cancer treatment. Within this landscape, TCR-engineered T-cell therapies have garnered significant attention, constituting a swiftly advancing and dynamic field. While CAR T therapies have demonstrated exceptional effectiveness in addressing B cell malignancies, there has been a diminishing focus on TCR T therapy. Nevertheless, interest in TCR T therapy is on the rise, particularly as CAR T trials have faced challenges in producing satisfactory responses in the context of solid cancers.
In January 2022, Immunocore reported the approval of Kimmtrak (tebentafusp-tebn) by the FDA for treating unresectable or metastatic uveal melanoma (mUM) in adult patients with HLA-A*02:01 positivity. The FDA’s decision was grounded in the findings of Immunocore’s Phase III IMCgp100-202 clinical trial, published in the New England Journal of Medicine on September 23, 2021. This pivotal trial, which included the largest Phase III cohort in mUM, assessed the overall survival benefit of Kimmtrak compared to the investigator’s choice (pembrolizumab, ipilimumab, or dacarbazine) in previously untreated mUM patients. The results revealed an unprecedented median overall survival benefit for Kimmtrak when used as a first-line treatment. In addition, Immunocore initiated a global early access program to ensure the widespread availability of Kimmtrak for mUM patients.
In September of 2023, Moderna revealed a strategic partnership for research and development aimed at leading the way in innovative therapies for cancer patients facing significant unmet medical needs. Additionally, in June 2023, Adaptimmune Therapeutics successfully finalized an all-stock transaction, merging with TCR² Therapeutics Inc. to establish a prominent T-cell therapy company focused on addressing solid tumors.
Regulatory Approvals and Challenges in Bringing TCR Therapies to the Market
The development and commercialization of TCR therapies represent a groundbreaking frontier in the field of immunotherapy, holding immense promise for treating various cancers and autoimmune diseases. However, the journey from laboratory innovation to market availability is fraught with regulatory complexities and challenges.
Regulatory approvals for TCR therapies involve navigating a dynamic landscape that requires careful scrutiny and adherence to stringent guidelines. The primary regulatory bodies, such as the FDA, the EMA, and their counterparts worldwide, necessitate comprehensive data demonstrating the safety, efficacy, and quality of TCR therapies. This demands rigorous preclinical and clinical studies to establish a robust scientific foundation, with particular emphasis on elucidating the therapeutic mechanism, identifying potential side effects, and ensuring the reproducibility of results.
One of the key challenges in bringing TCR therapies to market lies in addressing the unique and often unpredictable nature of immune responses. TCR therapies involve the genetic modification of a patient’s T cells to recognize and attack specific antigens, such as cancer cells. Ensuring the precision and specificity of these engineered T cells while minimizing off-target effects is a complex task. Additionally, the potential for severe adverse reactions, including cytokine release syndrome and neurotoxicity, necessitates careful risk assessment and management strategies.
Furthermore, the manufacturing process for TCR therapies introduces another layer of challenges. Developing scalable and reproducible manufacturing techniques that comply with regulatory standards is crucial for ensuring a consistent and reliable supply of these advanced therapies.
Ongoing Research and Clinical Trials: What Does the Future Hold for TCR Therapy?
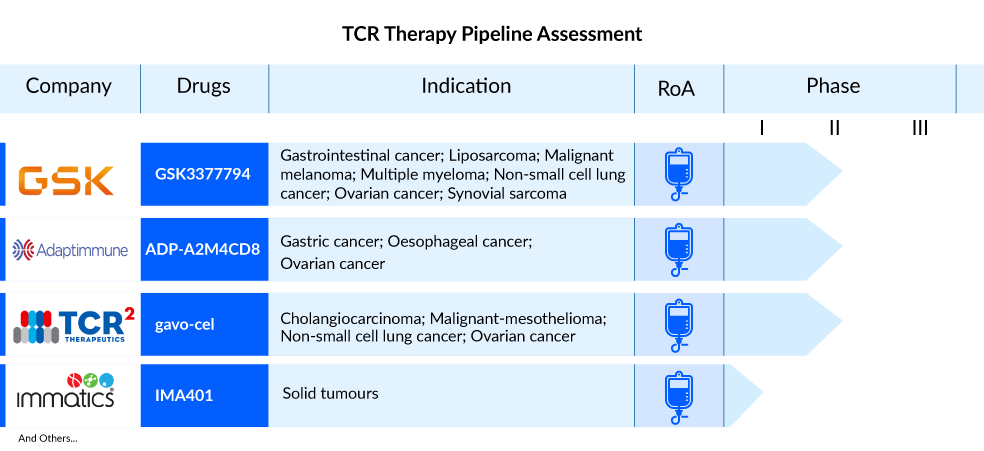
The landscape of the TCR therapy market is poised for transformation, as companies worldwide are actively engaged in the advancement of novel TCR therapy solutions to address various medical conditions. Major players with their lead assets, including GlaxoSmithKline (GSK3377794), Adaptimmune Therapeutics (afamitresgene autoleucel, ADP-A2M4CD8), Immatics (IMA401, IMA201, IMA202, IMA203), TCR² Therapeutics (gavo-cel), and others, are dedicated to the development of TCR therapy for treating diverse indications such as synovial sarcoma and myxoid/round cell liposarcoma, 2L+ non-small cell lung cancer, relapsed or refractory HPV-16-positive cancers, ovarian cancers, among others.
GSK3377794, developed by GlaxoSmithKline, represents a T-cell receptor therapy (TCR-T) that utilizes the body’s immune system to create a personalized treatment approach. The process involves extracting T-cells from the patient, genetically modifying them to express a T-cell receptor targeting the NY-ESO-1 antigen present in various solid tumors. This therapy has gained accelerated development status following European PRIME and FDA breakthrough designations and is classified as a registrational trial.
Additionally, GlaxoSmithKline is conducting a Phase II trial for 2L+ non-small cell lung cancer. The company is also actively engaged in two Phase I trials involving GSK3901961, featuring engineered TCR T-cells co-expressing the CD8a cell surface receptor targeting NY-ESO-1, and GSK3845097, comprising engineered TCR T-cells co-expressing the dnTGF-βRII cell surface receptor targeting NY-ESO-1, both with distinct mechanisms of action.
TCR² Therapeutics is in the process of developing gavo-cel, formerly known as TC-210, with the aim of targeting mesothelin-positive solid tumors. The company is currently engaged in a Phase I/II trial focusing on four specific indications: NSCLC, ovarian cancer, malignant pleural/peritoneal mesothelioma (MPM), and cholangiocarcinoma. Preliminary data from the ongoing Phase I/II clinical trial, particularly in the dose escalation phase, indicates consistent positive outcomes for gavo-cel. Almost every patient (15 out of 16) experienced tumor regression, and there was an 81% disease control rate (DCR).
In October 2021, TCR² Therapeutics announced a collaboration agreement with Bristol Myers Squibb to assess gavo-cel in combination with Opdivo (nivolumab) and Yervoy (ipilimumab) in a planned Phase II clinical trial targeting treatment-refractory mesothelin-expressing solid tumors. The FDA has granted Orphan Drug Designation for gavo-cel in the treatment of mesothelioma and cholangiocarcinoma, and the company intends to seek FDA Fast Track designation for gavo-cel.
Immatics is actively engaged in the development of targeted immunotherapies, placing a particular focus on addressing solid tumors using two distinct therapeutic approaches. These include Adoptive Cell Therapies (ACT), which encompass autologous (ACTengine) and allogeneic (ACTallo) product categories, as well as antibody-like TCR Bispecifics, also known as T cell Engaging Receptors (TCER). The company’s portfolio consists of seven therapeutic programs, with three currently undergoing clinical trials and five in the preclinical development phase. Alongside its proprietary pipeline, Immatics collaborates with renowned partners such as Bristol Myers Squibb, GlaxoSmithKline, and Genmab to expand therapeutic programs covering ACT and Bispecifics. Notably, Immatics is working in collaboration with Bristol Myers Squibb on the development of TCER IMA401, targeting MAGEA4 and MAGEA8, with the trial initiated in May 2022. The company’s TCR therapy clinical trial roster includes three proprietary TCR-T candidates: IMA203, IMA201, and IMA202.
Beyond Cancer: Exploring TCR Therapy in Infectious Diseases and Autoimmune Disorders
Traditionally associated with cancer treatment, TCR therapy has emerged as a promising avenue for addressing a spectrum of medical challenges beyond malignancies. This groundbreaking approach harnesses the power of the immune system, particularly T cells, to target and eliminate abnormal cells. While initially developed to combat cancerous cells, researchers are increasingly turning their attention to the potential applications of TCR therapy in infectious diseases and autoimmune disorders.
In infectious diseases, the adaptability of TCR therapy proves advantageous. The therapy’s capacity to reprogram T cells enables them to recognize and attack specific pathogens, offering a tailored and dynamic response to evolving infectious agents. This precision has the potential to revolutionize the treatment landscape, providing a more effective and targeted alternative to conventional antimicrobial strategies.
Moreover, the exploration of TCR therapy in autoimmune disorders holds great promise. By fine-tuning the immune response, researchers aim to modulate T-cell activity to distinguish between self and foreign entities, mitigating the aberrant immune reactions responsible for autoimmune conditions. This avenue of research signals a paradigm shift in the approach to autoimmune disorders, offering hope for novel and more effective therapeutic interventions that address the underlying causes of these complex conditions.
As research into TCR therapy expands beyond its original scope in cancer treatment, the potential to reshape the landscape of infectious diseases and autoimmune disorders becomes increasingly apparent. The intersection of immunology, genetics, and precision medicine holds the key to unlocking the full therapeutic potential of TCR therapy, ushering in a new era of targeted and personalized medicine for a diverse array of medical challenges.

Downloads
Article in PDF
Recent Articles
- Business CockTail
- Norlase’s LYNX Receives FDA and CE Approval for Clinical Use; FDA Approves Imperative Care’s Zoom...
- Acute Myeloid Leukemia: The Battle, The Breakthroughs, The Future
- Merck’s Keytruda sales; Valeant on name change; Pfizer – BMS; Amgen puts Repatha outcomes for deal
- AstraZeneca’s Imfinzi Shows Positive Results; Novartis Announces Results of Tislelizumab; FDA Gra...



