Roche’s TECENTRIQ HYBREZA: Setting New Standards as the First Subcutaneous Anti-PD-(L)1 Drug
Sep 23, 2024
Better late than never for FDA approval of the first subcutaneous PD-L1 inhibitor—Roche’s TECENTRIQ HYBREZA—after manufacturing delays disrupted the company’s initial launch plans last year.
The FDA was originally set to decide on TECENTRIQ’s under-the-skin formulation in September, but Roche’s delivery technology partner, Halozyme Therapeutics, noted that updates to the drug’s manufacturing processes were required. According to Roche, these adjustments were made to meet the FDA’s evolving requirements and were expected to be completed in 2023 to enable a 2024 launch. The formulation received its first approval in the UK last year.
On 13 September 2024, the FDA approved TECENTRIQ HYBREZA (atezolizumab and hyaluronidase-tqjs), marking it as the first and only PD-(L)1 inhibitor available for subcutaneous injection in the United States. Unlike the standard intravenous (IV) infusion of TECENTRIQ, which typically takes 30-60 minutes, TECENTRIQ HYBREZA can be administered subcutaneously in about seven minutes. It will be accessible for all the IV indications of TECENTRIQ approved for adults in the US, including certain lung, liver, skin, and soft tissue cancers.
Downloads
Article in PDF
Recent Articles
- Merck’s Keytruda Wins Another FDA Approval; Sanofi Pauses Trial of Myasthenia Gravis Drug, tolebr...
- FDA Grants Priority Review to BMS’ Luspatercept; Teva and MedinCell’s Risperidone FDA Approval; B...
- ESMO Asia 2022: Role of EGFR and ALK mutations in the East Asian Lung Cancer Market
- Nkarta’s Anti-CD19 Allogeneic CAR-NK Cell Therapy, NKX019; Eisai Presents Results of lecanemab fo...
- Novel Mutation-Targeting Therapies in the Horizon to Relieve the Global Healthcare Burden NSCLC P...
Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development, stated, “TECENTRIQ HYBREZA’s subcutaneous administration provides both patients with various types of cancer and their doctors with increased flexibility and options for treatment delivery. We are excited to launch this new subcutaneous formulation, which builds upon the proven safety and effectiveness of intravenous TECENTRIQ, offering a faster and more accessible treatment alternative.”
TECENTRIQ HYBREZA combines TECENTRIQ with Halozyme Therapeutics’ Enhanze drug delivery system. TECENTRIQ is a monoclonal antibody targeting the PD-L1 protein. It binds to PD-L1 on tumor cells and immune cells within tumors, blocking interactions with PD-1 and B7.1 receptors. This inhibition of PD-L1 can potentially reactivate T cells, though it may also impact normal cells.
The Enhanze technology uses a proprietary enzyme called recombinant human hyaluronidase PH20 (rHuPH20). This enzyme temporarily breaks down hyaluronan—a natural sugar chain—in the subcutaneous tissue, increasing tissue permeability. This allows TECENTRIQ to be rapidly distributed and absorbed into the bloodstream.
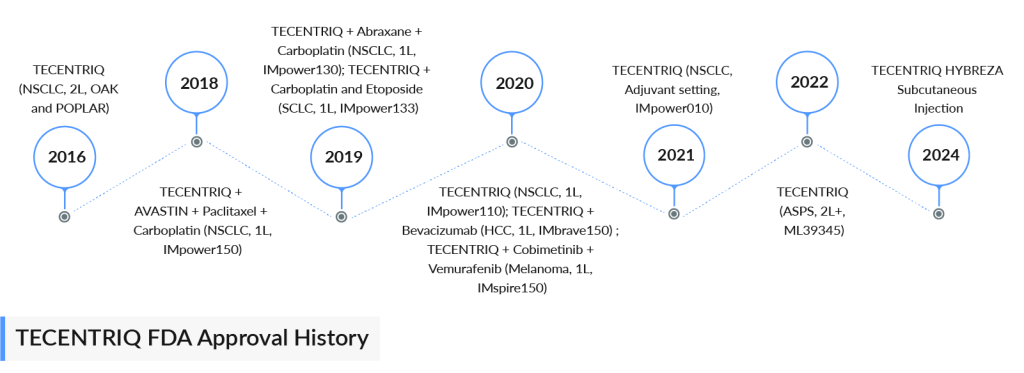
TECENTRIQ is authorized to treat some of the most aggressive and challenging cancers. It was the first immunotherapy approved for early-stage NSCLC, SCLC, and hepatocellular carcinoma. TECENTRIQ is also approved globally, either alone or combined with targeted therapies and/or chemotherapies, for various metastatic cancers, including NSCLC, certain metastatic urothelial cancers (mUC), PD-L1-positive metastatic TNBC, BRAF V600 mutation-positive advanced melanoma, and alveolar soft part sarcoma (ASPS). Additionally, TECENTRIQ is available in a subcutaneous formulation in 50 countries (marketed as TECENTRIQ SC outside the US), with its indications aligning with those of TECENTRIQ IV.
The FDA approval of Roche’s TECENTRIQ HYBREZA is based on crucial data from the Phase IB/III IMscin001 study and the Phase II IMscin002 study. IMscin001 is a Phase IB/III global, multicenter, randomized trial assessing the pharmacokinetics, safety, and efficacy of TECENTRIQ HYBREZA versus TECENTRIQ IV in patients with previously treated locally advanced or metastatic non-small cell lung cancer who have failed prior platinum-based therapy.
The study included 371 participants. It achieved its primary endpoints, showing similar blood levels of TECENTRIQ during dosing intervals based on pharmacokinetic measurements like observed serum Ctrough and model-predicted area under the curve. The efficacy, measured by objective response rate, progression-free survival, overall survival, and duration of response, was comparable between the SC and IV treatment groups and aligned with the known profile of TECENTRIQ IV. The safety profile of TECENTRIQ HYBREZA was also similar to that of TECENTRIQ IV.
IMscin002 is a Phase II global crossover trial assessing patient preference between the subcutaneous (SC) and intravenous (IV) forms of TECENTRIQ. The trial included 179 patients, such as those with PD-L1-positive resected Stage II-IIIB NSCLC who had completed adjuvant platinum-based chemotherapy without signs of disease recurrence, as well as untreated patients with PD-L1-high Stage IV NSCLC. The trial achieved its primary goal, revealing that 71% of participants favored the SC formulation, 21% preferred the IV formulation, and 8% had no preference. Furthermore, 79% chose TECENTRIQ HYBREZA to finish their treatment after experiencing both forms of TECENTRIQ. The study demonstrated that switching between TECENTRIQ HYBREZA and TECENTRIQ IV was well tolerated and did not introduce new safety concerns.
The delivery technology has contributed to making administration easier for other prominent cancer treatments, such as Johnson & Johnson’s DARZALEX FASPRO and potentially Bristol Myers Squibb’s OPDIVO, which is still awaiting approval for a subcutaneous form.
TECENTRIQ HYBREZA has become the first subcutaneous checkpoint inhibitor approved by the FDA, surpassing OPDIVO, which is still under review. The FDA’s decision on the new OPDIVO formulation is anticipated later this year.
TECENTRIQ, approved in 2016, generated CHF 3.77 billion ($4.44 billion) in sales for 2023. Despite tough competition from major players like Merck’s KEYTRUDA in NSCLC, Roche maintains a strong position in the US.
In summary, as the first subcutaneous anti-PD-(L)1 drug, TECENTRIQ HYBREZA has set a new benchmark in cancer therapy, offering patients a groundbreaking approach with its convenient, less invasive administration. This innovative delivery method not only enhances patient comfort but also aligns with the evolving demand for more accessible treatment options. As competitors enter the arena, TECENTRIQ HYBREZA’s early entry and established efficacy will serve as a formidable advantage, ensuring it remains a top choice among oncologists and patients alike.
In a rapidly advancing field, TECENTRIQ HYBREZA is poised to maintain its leadership position by continuously leveraging its pioneering status and superior clinical outcomes. With ongoing research and development aimed at expanding its indications and improving patient experiences, TECENTRIQ HYBREZA is set to outpace emerging competitors. Its unique formulation and proven track record solidify its role as a trusted and innovative solution in the fight against cancer, promising continued success and growth in the coming years.

Downloads
Article in PDF
Recent Articles
- Tesaro’s Zejula; Mylan on EpiPen; Ohio sues drugmakers; ATUM, Horizon Discovery Announce deal
- FDA grants accelerated approval to pembrolizumab
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Takeda and AC Immune’s Alzheimer’s Deal; Eli Lilly’s Donanemab FDA Review; Bristol Myers Sq...
- CDK 4/6 Inhibitors- The Changing Paradigm for Breast Cancer Treatment