TEVIMBRA’s Winning Streak — BeiGene Secures New Indication for PD-1 Drug
Mar 07, 2025
On March 3, 2025, BeiGene secured another approval for its anti-PD-1 antibody, TEVIMBRA, as the FDA granted clearance for its use in combination with platinum-based chemotherapy as a first-line treatment for adults with unresectable or metastatic esophageal squamous cell carcinoma (ESCC) whose tumors express PD-L1.
TEVIMBRA is also approved in the US as a standalone treatment for adult patients with unresectable or metastatic esophageal squamous cell carcinoma who have previously received systemic chemotherapy without a PD-(L)1 inhibitor. Additionally, it is authorized in combination with chemotherapy as a first-line treatment for adults with gastric and gastroesophageal junction (G/GEJ) cancers.
TEVIMBRA is a specially engineered humanized immunoglobulin G4 (IgG4) monoclonal antibody targeting programmed cell death protein 1 (PD-1) with high affinity and specificity. It is designed to reduce interaction with Fc-gamma (Fcγ) receptors on macrophages, enhancing the immune system’s ability to recognize and attack tumors.
Downloads
Article in PDF
Recent Articles
- Antibody-Drug Conjugates Market Outlook, 2015 Report
- The next generation of ‘weaponized antibody’ therapies
- Insights into the Evolving Landscape of Antibody-Drug Conjugate (ADC) & the Key Companies in...
- Treatment for esophageal cancer; FDA bans soaps; Dr Reddy’s Launches; Cipla Gets Approval
- Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies
Dr. Nataliya Uboha, Associate Professor at the University of Wisconsin’s Carbone Cancer Center, stated that the approval of TEVIMBRA alongside chemotherapy for adult patients with ESCC broadens the available first-line treatment options. She emphasized the urgent need for effective therapies for ESCC and highlighted that TEVIMBRA has demonstrated improved outcomes for this patient group.
As a cornerstone of BeiGene’s solid tumor portfolio, TEVIMBRA has demonstrated potential across various cancer types and disease settings. Its global clinical development program has enrolled nearly 14,000 patients in 66 trials across 34 countries and regions, including 20 pivotal registration-enabling studies. Currently, TEVIMBRA is approved in over 42 countries, with more than 1.3 million patients treated worldwide. In the past year, TEVIMBRA generated $621 million in revenue, contributing significantly to BeiGene’s total earnings of $3.8 billion.
Esophageal cancer ranks as the sixth leading cause of cancer-related deaths worldwide, with esophageal squamous cell carcinoma being the predominant histologic subtype, comprising approximately 90% of cases. The 7MM accounted for approximately 77K diagnosed incident cases of esophageal cancer in 2023, as per DelveInsight analysis.
The highest number of diagnosed incident cases of esophageal cancer were observed in Japan, and the second highest number of diagnosed incident cases of esophageal cancer were observed in the US, which was followed by the UK. This disease progresses rapidly, and over two-thirds of patients are diagnosed at an advanced or metastatic stage. For those with distant metastases, the five-year survival rate remains alarmingly low at less than 6%.
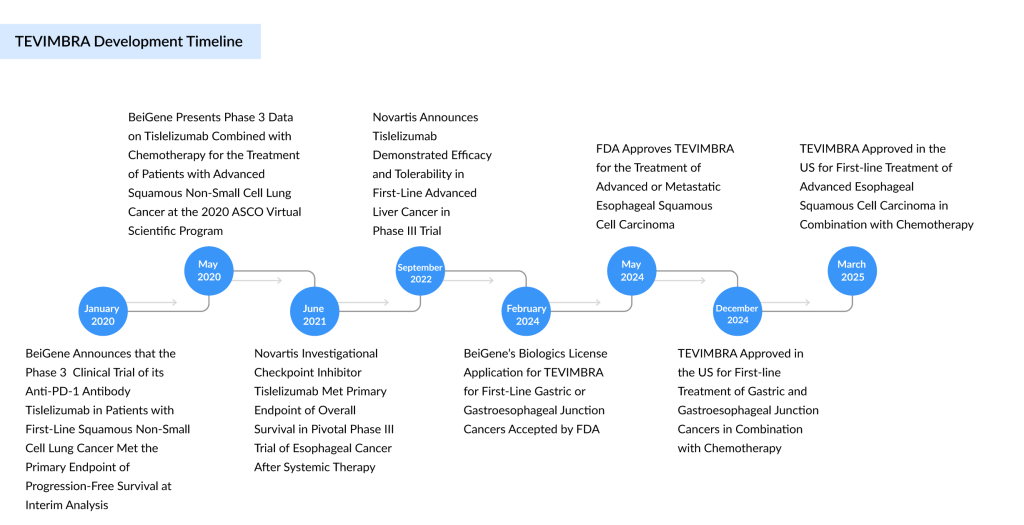
The expanded indication approval of TEVIMBRA is supported by findings from BeiGene’s RATIONALE-306 (NCT03783442) trial, a global, randomized, placebo-controlled, double-blind Phase III study assessing the efficacy and safety of TEVIMBRA combined with platinum-based chemotherapy as a first-line treatment for adults (n=649) with unresectable, locally advanced recurrent, or metastatic ESCC. The trial achieved its primary endpoint, showing a statistically significant improvement in overall survival (OS) for patients receiving TEVIMBRA plus chemotherapy compared to those given a placebo with chemotherapy.
Exploratory analyses suggested that the survival benefit in the intent-to-treat (ITT) population was largely driven by patients with PD-L1 expression ≥1. Among PD-L1 positive (≥1) patients (n=481), the median OS was 16.8 months for those treated with TEVIMBRA and chemotherapy versus 9.6 months for the placebo group (HR: 0.66, [95% CI: 0.53, 0.82]), reflecting a 34% reduction in mortality risk. These findings represent a significant advancement in first-line treatment for ESCC.
Mark Lanasa, M.D., Ph.D., Chief Medical Officer for Solid Tumors at BeiGene, highlighted the significance of the FDA’s decision, stating, “Receiving our third FDA approval in under a year underscores our commitment to pioneering innovative treatments and addressing critical gaps in cancer care. The approval of TEVIMBRA as a first-line therapy for advanced esophageal squamous cell carcinoma represents a major advancement in meeting the needs of patients facing this difficult disease. We deeply appreciate the dedication and perseverance of the patients, clinicians, and researchers who have contributed to this progress.”
The safety profile of TEVIMBRA in combination with chemotherapy was assessed in the same trial. The most common serious adverse reactions (≥2%) included pneumonia, dysphagia, diarrhea, fatigue, and esophageal stenosis, while frequently reported adverse reactions (≥20%) comprised anemia, fatigue, reduced appetite, nausea, constipation, weight loss, diarrhea, peripheral sensory neuropathy, vomiting, and stomatitis.
The FDA’s approval follows a separate label expansion granted in late 2024 for several types of gastric and gastroesophageal carcinoma. The company is also investigating TEVIMBRA across multiple cancer types, including hepatocellular cancer, where a Phase III trial reported positive results in 2022. Furthermore, the company anticipates an imminent approval for first-line ESCC treatment in Japan. Additionally, it is conducting the Phase III HERIZON-GEA-01 trial in partnership with Jazz Pharmaceuticals to evaluate TEVIMBRA for HER2-positive advanced or metastatic gastroesophageal adenocarcinoma.
BeiGene’s pipeline is largely focused on cancer treatments, featuring Ociperlimab, an anti-TIGIT antibody for non-small-cell lung cancer, and BG-685011, a CDK2 inhibitor targeting solid tumors. Additionally, the company is expanding into a new domain with two antibody-drug conjugates, both currently in Phase I trials, for various solid tumors.
In addition to BeiGene, several other companies, such as Hoffmann-La Roche, Merck Sharp & Dohme LLC, AstraZeneca, and others, are also working with their lead assets in different stages of development for esophageal squamous cell carcinoma.
The upcoming launch of therapies from various companies is expected to significantly enhance the esophageal cancer therapeutic landscape. These advancements will not only expand treatment options for patients but also drive innovation in the field. However, this influx of new treatments will simultaneously intensify competition for BeiGene’s TEVIMBRA, challenging its market position.
As multiple players enter the space, differentiation through efficacy, safety profiles, and pricing strategies will be crucial for maintaining a competitive edge. BeiGene may need to strengthen its market strategy by focusing on clinical advantages, strategic partnerships, or expanded indications to sustain TEVIMBRA’s growth. Additionally, increased competition could lead to pricing pressures and the need for continuous innovation to meet evolving patient and physician expectations.

Downloads
Article in PDF
Recent Articles
- 7 Companies at the Forefront of Trispecific Antibody Development
- Bristol-Myers Squibb’s Opdivo Approval; Mirati’s KRAS-inhibitor Adagrasib; J&J and Legend Bi...
- OPDIVO vs. KEYTRUDA: The Battle for Japan’s PD-1 Inhibitor Market Dominance
- Antibody-drug Conjugates in Oncology: An Overview of the Current and Future Treatment Landscape
- GeneDx secures $92M funding; BioTech Innovations partners with Novartis for R&D collaboration