Servier’s Tibsovo Opens The Door To A New Era In The Battle Against Myelodysplastic Syndromes
Nov 13, 2023
Servier’s Tibsovo (ivosidenib tablets) has received an expanded indication from the FDA, allowing its utilization in patients diagnosed with relapsed or refractory myelodysplastic syndromes that exhibit an IDH1 mutation. This new approval represents the fifth for Tibsovo, which is already recognized for its efficacy in acute myeloid leukemia (AML) and cholangiocarcinoma. This recent regulatory endorsement establishes Tibosovo as the premier and sole approved targeted therapy for a defined subset of relapsed or refractory myelodysplastic syndromes patients based on their molecular characteristics.
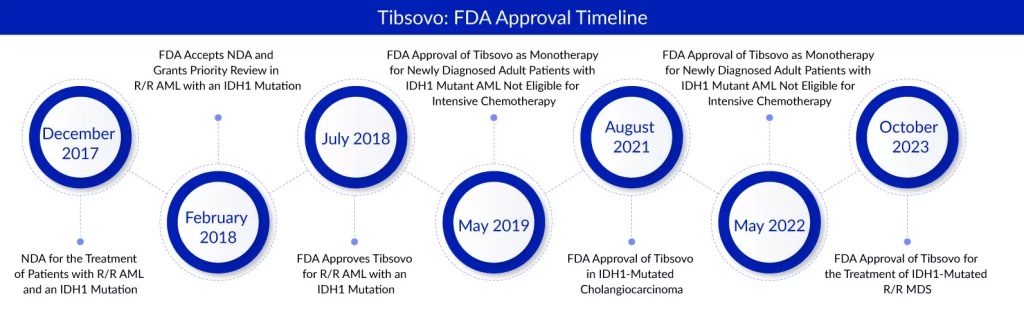
TIBSOVO, a precision medicine designed to target the specific mutation isocitrate dehydrogenase 1 (IDH1), has obtained approvals across five global indications. These approvals span multiple regions, including the U.S., European Union, Australia, and China. In the United States, TIBSOVO is authorized for various treatments. It is approved for treating adults with relapsed or refractory AML featuring IDH1 mutations, both as a standalone therapy and in combination with azacitidine for newly diagnosed AML patients over 75 years old or those with medical conditions unsuitable for intensive induction chemotherapy. Furthermore, it is approved as a monotherapy for adults with relapsed or refractory MDS featuring IDH1 mutations, along with patients previously treated for IDH1-mutated cholangiocarcinoma. Servier has granted CStone a co-exclusive license for development and an exclusive license agreement for commercializing TIBSOVO in Mainland China, Taiwan, Hong Kong, Macao, and Singapore.
The FDA awarded TIBSOVO Breakthrough Therapy designation for treating adult patients with relapsed or refractory myelodysplastic syndromes bearing the IDH1 mutation. This designation facilitated a Priority Review, expediting the assessment process for medications that offer substantial advancements in treatment effectiveness or safety for severe conditions. Accompanying Tibsovo’s recent approval, the FDA has also sanctioned the Abbott Laboratories’ RealTime IDH1 Assay. This diagnostic tool is designed to complement the drug by identifying suitable patients for Tibsovo treatment.
Downloads
Article in PDF
Recent Articles
- Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announce...
- Precigen’s PRGN-3006; Yescarta Approved as a First CAR T-cell Therapy for R/R LBCL; Biogen ...
- Agios’ PYRUKYND SNDA Accepted by FDA for Thalassemia; BridgeBio’s BBO-8520 Gets FDA Fast Track fo...
- Daiichi Sankyo’s Intravenous Iron Replacement Therapy; ANeuroTech’s Adjunctive Anti-depression Dr...
- FDA Grants Fast Track for Lin BioScience’s LBS-007; Alnylam’s AMVUTTRA sNDA Under Review; FDA App...
Myelodysplastic syndromes (MDS) encompass a collection of rare blood cancers resulting from genetic mutations in hematopoietic stem cells, impeding their normal development into fully functional blood cells. As per Delveinsight’s analysis, the total incident population of myelodysplastic syndrome in the 7MM was around 42K in 2022, growing at a 1% CAGR during the study period (2019–2032). As per the estimates, the United States accounted for approximately 21K incident cases of myelodysplastic syndrome in 2022, which was the highest among the 7MM.
Arjun Prasad, Head of Commercial at Servier Pharmaceuticals, emphasized, Servier takes pride in spearheading the field of mutant IDH inhibition, persistently innovating to assist patients dealing with challenging, hard-to-treat cancers. The latest FDA approval for TIBSOVO, serving as the sole targeted therapy for individuals with IDH1-mutated relapsed or refractory myelodysplastic syndromes, further underscores our commitment to making significant progress in addressing crucial unmet medical needs. Our aim remains steadfast in providing tailored treatments to the appropriate patients precisely when required.
The IDH1 gene encodes the isocitrate dehydrogenase 1 enzyme, crucial in converting isocitrate into α-ketoglutarate, a vital component in energy production under normal circumstances. However, in the presence of pathological mutations, this enzyme produces 2-hydroxyglutarate (2-HG), an identified oncometabolite. Tibsovo, developed by Servier, specifically targets and inhibits the mutated IDH1 enzymes, resulting in a decrease in 2-HG production. The drug received its initial approval in July 2018 for patients with relapsed or refractory acute myeloid leukemia (R/R AML) carrying the same susceptible IDH1 mutation.
“The expanded application of targeted therapy for cancers characterized by IDH mutations represents a significant advancement in treatment options for patients falling within this precise molecular subtype,” commented Dr. Amir Fathi, a specialist in myeloid malignancies and an expert in hematology and oncology. “The latest approval for IDH1-mutated relapsed or refractory myelodysplastic syndromes underscores the critical role of mutational testing in guiding treatment strategies, offering the potential to enhance patient outcomes.”
This regulatory clearance for Tibosovo was substantiated by evidence derived from a pivotal Phase I open-label investigation involving 18 R/R MDS individuals harboring IDH1 mutations. The administered treatment using Servier’s medication resulted in a noteworthy complete remission rate of 38.9%, achieved within a median period of 1.9 months. As of the data cutoff, the median duration of complete response had not been determined. The study displayed an impressive 83.3% objective response rate, and the median overall survival was recorded at 35.7 months.
Out of the nine patients previously reliant on red blood cell or platelet transfusions, six no longer needed these transfusions following the administration of Tibsovo. The oral medication displayed no new safety issues of concern, and the reported adverse events were consistent with those previously documented. Tibsovo’s labeling includes a boxed warning for differentiation syndrome in both MDS and AML, emphasizing its potential fatality. Servier remains dedicated to the continued development of ivosidenib for various solid tumor indications, exploring its efficacy as a standalone MDS treatment or as part of a combination therapy.
“This new approval for TIBSOVO brings hope to the MDS community,” stated Tracey Iraca, Executive Director of the MDS Foundation. “Until now, there were no targeted therapies approved for relapsed or refractory MDS patients carrying the IDH1 mutation. We extend our gratitude to the study participants, their families, caregivers, Servier’s researchers, and the clinical investigators involved in this study for contributing to the introduction of a much-needed treatment option for these patients.”
Apart from this, several other companies are conducting trials for myelodysplastic syndrome treatment including Fibrogen (Roxadustat (FG-4592), AbbVie (Venclexta), Gilead Sciences (Magrolimab), Novartis (Sabatolimab), Syros Pharmaceuticals (Tamibarotene), Geron Corporation (imetelstat), KaryopharmTherapeutics/Antengene Corporation (Eltanexor), Bristol Myers Squibb (Enasidenib), Vincerx Pharma (VIP943), and others. The anticipated launch of therapies by these companies will give fierce competition to Servier’s Tibsovo in myelodysplastic syndrome treatment space.

Downloads
Article in PDF
Recent Articles
- Gilead to buy Forty Seven; Akrevia Therapeutics unveils new identity; FDA approved pyrimethamine
- Daiichi Sankyo’s Intravenous Iron Replacement Therapy; ANeuroTech’s Adjunctive Anti-depression Dr...
- Agios’ PYRUKYND SNDA Accepted by FDA for Thalassemia; BridgeBio’s BBO-8520 Gets FDA Fast Track fo...
- Gilead’s Magrolimab Plus Azacitidine for MDS; FDA Approveds VANFLYTA for Newly Diagnosed AML; FDA...
- Myelodysplastic Syndromes (MDS): Key Pharma Players Unveiling Updated Findings at ASH 2021