Top 8 Breakthrough Gene Therapies for Retinitis Pigmentosa Treatment
Jul 26, 2024
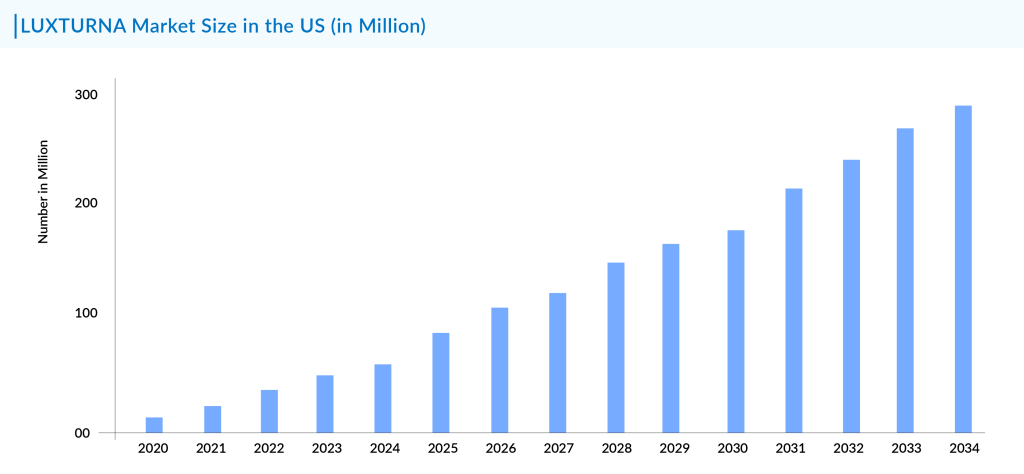
Gene therapy is becoming a promising solution for retinal degenerative diseases, as the retina offers an excellent setting for studying and treating eye conditions. Importantly, it was the first tissue to receive approved gene therapy for genetic disorders in the United States. To date, only one retinitis pigmentosa treatment regimen, LUXTURNA (developed by Spark Therapeutics), has been approved in the United States.
LUXTURNA, the only FDA-approved gene therapy for retinitis pigmentosa treatment, also known as voretigene neparvovec-rzyl, is a one-time gene therapy designed for patients with biallelic RPE65 mutation-associated retinal dystrophy. It received approval from the FDA in December 2017.
This therapy is notable as the first FDA-approved gene therapy for a genetic condition, the first and only pharmacological treatment for an inherited retinal disease, and the first AAV vector gene therapy approved in the US. Spark Therapeutics developed and commercialized the drug in the United States, while Novartis is marketing LUXTURNA gene therapy in Europe under a licensing agreement for development, registration, and commercialization outside the US. The FDA’s advisory panel praised LUXTURNA’s approval in October 2017. In June 2023, Japan’s MHLW also approved LUXTURNA for treating inherited retinal dystrophies (IRDs) caused by biallelic RPE65 mutations.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Merck’s Keytruda Wins Another FDA Approval; Sanofi Pauses Trial of Myasthenia Gravis Drug, tolebr...
- Cannabidiol trial; MyoKardia aims; LDL drug data
- Fourth FDA Approval for AbbVie’s Vraylar; FDA Approves Ferring’s Adstiladrin for NMIBC; Merck and...
- Abingworth & Alebund’s Finacial Closing; Pfizer/BioNTech COVID-19 Vaccine Expanded Use...
- EMA to relocate to Amsterdam; Roche’s prospects; Amgen’s Humira Biosimilar delayed; Purdue’s opio...
Before LUXTURNA, no gene therapies had been approved for eye disorders, leaving a notable gap in treatment options for individuals with inherited retinal diseases. The success of LUXTURNA exemplifies the transformative potential of retinitis pigmentosa gene therapy, demonstrating promising outcomes for patients with retinitis pigmentosa and pioneering the role of viral vector-based treatments.
Gene and cell therapies are crucial and target various genes such as RHO, USH2A, and RPGR. USH2A stands out as a key focus, with two oligonucleotide candidates playing significant roles in the retinitis pigmentosa treatment pipeline. Optogenetics offers a novel retinitis pigmentosa gene therapy that addresses the constraints of conventional methods. It functions independently of particular genes and is effective for advanced diseases marked by significant photoreceptor loss.
The emerging retinitis pigmentosa pipeline is full of late-stage, mid-stage, and early-stage drugs. As per DelveInsight’s estimates, the potential gene therapies for retinitis pigmentosa treatment that can mark a significant change in the coming years include Botaretigene sparoparvovec (Johnson & Johnson Innovative Medicine/MeiraGTx), AGTC-501 (Beacon Therapeutics), MCO-010 (Nanoscope Therapeutics), GS030 (Gensight Biologics), 4D-125 (4D Molecular Therapeutics), CTx-PDE6b (Coave Therapeutics), OCU400 (Ocugen), and BS01 (Bionic Sight).
Here take a closer look at these gene therapies for retinitis pigmentosa treatment in detail.
Johnson & Johnson Innovative Medicine/MeiraGTx’s Botaretigene sparoparvovec
Molecule Type: AAV5 Gene Therapy
Phase: Phase III
Botaretigene sparoparvovec (bota-vec) is intended to address X-linked retinitis pigmentosa, specifically the form caused by mutations in the RPGR gene’s eye-specific segment, known as RPGR open reading frame 15 (RPGR ORF15). This gene is crucial for the function of both rod and cone photoreceptors. The Phase I/II trials of bota-vec, involving both adults and children, have concluded, and the Phase III Lumeos trial finished enrolling participants in 2023.
The retinitis pigmentosa treatment drug demonstrated a favorable safety profile, with the proof-of-concept study showing improvements in retinal sensitivity, visual function, and overall vision. These results were highlighted in a late-breaking presentation by Professor Michel Michaelides at the American Academy of Ophthalmology (AAO) 2022 Annual Meeting’s Retina Subspecialty Day. The drug has received Fast Track and Orphan Drug designations from the FDA and PRIME, ATMP, and Orphan Medicinal Product designations from the EMA. It is currently in the Phase III development stage for X-linked retinitis pigmentosa treatment.
In December 2023, MeiraGTx Holdings revealed an agreement to acquire the remaining shares of bota-vec, a treatment for XLRP, from Johnson & Johnson Innovative Medicine, a subsidiary of Johnson & Johnson. The deal also includes a commercial supply agreement and a technology transfer agreement for the manufacturing of bota-vec.
Beacon Therapeutics’ AGTC-501
Molecule Type: AAV2 Gene Therapy
Phase: Phase II/III
AGTC-501 is a gene therapy program now undergoing Phase II/III clinical trials for X-linked retinitis pigmentosa treatment. It was acquired when Syncona purchased AGTC in November 2022. XLRP is mainly due to mutations in the RPGR gene, which affects the retinitis pigmentosa GTPase regulator. Unlike other retinitis pigmentosa treatments, AGTC-501 delivers the complete RPGR protein, targeting the full range of photoreceptor damage associated with XLRP, including both rod and cone cell loss.
Recently, Beacon Therapeutics Holdings Limited has revealed that it has secured $170 million in Series B funding. Forbion spearheaded the investment round, which also saw participation from current investors Syncona Limited, Oxford Science Enterprises, and the University of Oxford, along with new contributions from TCGX and Advent Life Sciences. The capital will be allocated to advance the clinical development of Beacon’s primary asset, AGTC-501, for XLRP and to produce Phase I/II clinical trial data for its dry AMD program.
Nanoscope’s MCO-010
Molecule Type: AAV2-delivered optogenetic gene therapy
Phase: II
Nanoscope’s MCO-010 gene therapy for retinitis pigmentosa employs a simple and proven intraocular injection method to introduce a gene that codes for the light-sensitive MCO protein into retinal cells. This approach aims to help retinal cells detect light, potentially restoring vision for individuals with retinitis pigmentosa or Stargardt disease. MCO-010 is currently in clinical trials for these two rare retinal disorders that lead to blindness.
Nanoscope Therapeutics Inc. reported favorable top-line results following the completion of the 2-year Phase IIb RESTORE randomized, controlled clinical trial for its leading program, MCO-010, a retinitis pigmentosa gene therapy designed for patients with severe, permanent vision loss due to advanced retinitis pigmentosa.
The trial achieved its main goal, showing a statistically significant improvement in best-corrected visual acuity (BCVA) at week 52 for both the high-dose (0.337 LogMAR; p=0.021) and low-dose (0.382 LogMAR; p=0.029) groups compared to the sham control group (0.050 LogMAR). This Phase IIb RESTORE trial is unique in retinal degenerative disease research for demonstrating a statistically significant improvement exceeding the clinically relevant BCVA > 0.3 LogMAR threshold.
MCO-010 has received FDA fast-track designations and FDA orphan drug designations for both retinitis pigmentosa cure and Stargardt disease treatment.
Gensight’s GS030
Molecule Type: AAV2 optogenetic gene therapy
Phase: I/II
GS030 represents an advanced approach combining two synergistic elements: a gene therapy product that encodes a light-sensitive channelrhodopsin protein, delivered using a specially modified AAV2 vector known as AAV2 7m8, and biomimetic goggles designed to activate the modified retinal cells. This system employs optogenetics, a method that transfers a gene encoding a light-sensitive protein into neuronal cells, enabling them to react to light. GS030 features a bio-engineered AAV2 vector that inserts the gene for this photosensitive protein (which we exclusively control in the field of optogenetics) into the nuclei of retinal ganglion cells (RGCs).
This gene imparts photoreceptive abilities to otherwise healthy and preserved RGCs, regardless of the specific genetic mutation responsible for photoreceptor degeneration. GS030 is currently undergoing a Phase I/II trial for non-syndromic retinitis pigmentosa treatment.
4D Molecular Therapeutics’ 4D-125
Molecule Type: AAV (4D-R100) gene therapy
Phase: I/II
4D-125 is an experimental genetic therapy that uses the R100 vector to treat XLRP caused by mutations in the RPGR gene. This candidate stands out because it employs the primate-adapted R100 vector for delivering the RPGR transgene directly to the retina, aiming for expression throughout the retina with just one dose. Currently, 4D-125 is being tested in a Phase I/II clinical trial for XLRP treatment. In January 2022, 4D Molecular Therapeutics announced that the FDA had granted Fast Track Designation for 4D-125 for the treatment of patients with IRDs due to defects in the RPGR gene, including XLRP.

Coave Therapeutics’ CTx PDE6B
Molecule Type: AAV5 gene therapy
Phase: I/II
CTx PDE6B is a gene therapy currently in clinical development that uses AAV5 to introduce a complete, non-mutated version of the human PDE6b gene into the subretinal space. This retinitis pigmentosa therapy aims to induce strong expression and production of functional PDE6b proteins in the photoreceptor cells, rods, and cones. By supplying these cells with the necessary protein, CTx PDE6B has the potential to significantly slow down or stop retinal degeneration in patients with PDE6ß deficiency. It is now being tested in a Phase I/II trial for retinitis pigmentosa treatment.
Ocugen’s OCU400
Molecule Type: AAV5 gene therapy
Phase: I/II
OCU400 (AAV-NR2E3) is an innovative gene therapy candidate with the potential to effectively restore retinal health and function in a variety of inherited retinal diseases (IRDs) that have different genetic backgrounds. It involves introducing a functional copy of the NR2E3 gene, which encodes a nuclear hormone receptor (NHR), into retinal cells using an AAV vector. As a powerful modifier gene, NR2E3 expression in the retina could help restore retinal balance, stabilize cells, and prevent photoreceptor degeneration. In studies with five distinct mouse models of retinitis pigmentosa, subretinal injection of AAV-NR2E3 successfully protected photoreceptors from further damage in various IRDs after the disease had begun. OCU400 is currently undergoing Phase I/II trials for retinitis pigmentosa treatment and Leber congenital amaurosis (LCA).
In December 2023, Ocugen announced that the FDA had granted RMAT designation to Ocugen’s investigational product OCU400 for the treatment of retinitis pigmentosa associated with RHO mutations. In December 2022, Ocugen announced that the FDA granted orphan drug designations to OCU400 for the treatment of retinitis pigmentosa and LCA.
Recently in June Ocugen announced that the first patient has been administered the treatment in its Phase III liMeliGhT clinical trial for OCU400. This Phase III trial is built on encouraging results from Phase I/II studies of OCU400, which showed positive trends in Best-Corrected Visual Acuity (BCVA), Multi-Luminance Mobility Testing (MLMT), and Low-Luminance Visual Acuity (LLVA) in treated eyes. Among the 18 subjects with retinitis pigmentosa, 89% showed either preservation or improvement in BCVA, LLVA, or MLMT scores from baseline. Additionally, 80% of subjects with RHO mutations experienced preservation or improvement in MLMT scores, and 78% of all subjects demonstrated preservation or improvement in MLMT scores in the treated eyes from baseline.
Bionic Sight’ BS01
Molecule Type: AAV gene therapy
Phase: I/II
BS01, a gene therapy vector, was developed at the Belfer Gene Therapy Core Facility at Weill Cornell Medicine. This facility supports both basic science and clinical research (GMP level) involving gene transfer vectors. BS01 is an experimental gene therapy designed for advanced retinitis pigmentosa. It works by delivering optogenetic proteins to the optic nerve, which converts light into neural signals that are then transmitted to the brain. Unlike other AAV-based therapies for retinitis pigmentosa treatment, BS01 includes a device created by Niremberg that utilizes the retina’s neural code. Initial results from the first four patients who received BS01, Bionic Sight’s experimental gene therapy for advanced retinitis pigmentosa, indicated that all of them were able to perceive light and motion, with two patients also detecting direction. BS01 is currently undergoing a Phase I/II trial for retinitis pigmentosa treatment.
Apart from these, several other gene therapies are also in the retinitis pigmentosa treatment pipeline. Retinitis pigmentosa companies such as Neurotech Pharmaceuticals (NT-501, Phase II), AbbVie (RST-001, Phase I/IIb), SparingVision (SPVN06, Phase I/II; SPVN20, Preclinical), STZ eyetrial (rAAV8.hPDE6A, Phase I/II), ViGeneron (VG901, Phase Ib), PYC Therapeutics (VP-001, Phase I), Ray Therapeutics (RTx-015, Preclinical), and others are evaluating their lead candidates in different stages of clinical development.
The future of treatments for retinitis pigmentosa cure looks encouraging. Retinitis pigmentosa clinical trials for gene and stem cell therapies have shown promising results in terms of safety and early signs of effectiveness. Gene therapy, in particular, is making rapid progress and is considered more effective, less invasive, and relatively safer in the short term compared to retinal transplantation.
While research into gene therapy aims to improve vision for those with retinitis pigmentosa, it faces challenges such as developing gene transfer technology and a shortage of suitable animal models. Like other retinitis pigmentosa treatments, gene therapy needs further refinement in terms of efficacy, safety, and long-term stability. However, with growing interest and active advancements in each therapeutic area, we are entering a new era in diagnosing and treating retinitis pigmentosa.

Frequently Asked Questions
The only FDA-approved one is LUXTURNA (voretigene neparvovec-rzyl) for patients with biallelic RPE65 mutations. It’s a one-time gene therapy designed to restore vision in that specific genetic subtype.
Are there any gene therapy treatments approved for retinitis pigmentosa? The only FDA-approved one is LUXTURNA (voretigene neparvovec-rzyl) for patients with biallelic RPE65 mutations. It’s a one-time gene therapy designed to restore vision in that specific genetic subtype.
What are some promising emerging gene therapies in the pipeline for retinitis pigmentosa?
How is retinitis pigmentosa inherited, and what genes are most commonly targeted by gene therapies? Retinitis pigmentosa (RP) is inherited in various ways: autosomal dominant, autosomal recessive, or X-linked. Key genes being targeted in the pipeline include RPGR, PDE6B, RHO, USH2A, and NR2E3.
Main challenges include safe delivery of the gene vector across the retina, achieving durable expression, dealing with advanced disease when photoreceptors are already lost, obtaining regulatory approval, and ensuring safety (immune response, off-target effects), as well as cost and manufacturing scale. Is there hope for patients without the RPE65 mutation? Many therapies in the pipeline aim to help patients with other genetic forms. For example, RPGR-based therapies (Botaretigene sparoparvovec, AGTC-501, 4D-125), or optogenetic therapies like GS030 are being developed to work irrespective of the specific photoreceptor mutation when some retinal structure remains.
Retinitis pigmentosa (RP) is inherited in various ways: autosomal dominant, autosomal recessive, or X-linked. Key genes being targeted in the pipeline include RPGR, PDE6B, RHO, USH2A, and NR2E3.
Main challenges include safe delivery of the gene vector across the retina, achieving durable expression, dealing with advanced disease when photoreceptors are already lost, obtaining regulatory approval, and ensuring safety (immune response, off-target effects), as well as cost and manufacturing scale.
Many therapies in the pipeline aim to help patients with other genetic forms. For example, RPGR-based therapies (Botaretigene sparoparvovec, AGTC-501, 4D-125), or optogenetic therapies like GS030 are being developed to work irrespective of the specific photoreceptor mutation when some retinal structure remains.
Downloads
Article in PDF
Recent Articles
- Reversing Ischemia with Gene therapy
- The Business Cocktail
- First Gene Therapy for Severe Hemophilia A; FDA Approves CellTrans’s Type 1 Diabetes Cellular The...
- DelveInsight’s Otolaryngology based Gene Therapy Reports
- Hemophilia B Market: How Pipeline Therapies are Transforming the Treatment Hemisphere?



