6 Emerging Treg Cell-based Therapies Shaping the Future of Immunotherapy
Dec 30, 2024
Table of Contents
Treg (regulatory T-cell) therapies utilize the immunosuppressive abilities of regulatory T-cells to address various conditions, such as autoimmune diseases, inflammatory disorders, and transplant rejection. These treatments take advantage of Tregs’ natural role in maintaining immune balance by suppressing overactive immune responses that could lead to tissue damage and preventing autoimmunity. Tregs accomplish this by regulating the activity of other immune cells, ensuring a balanced immune system that is crucial for overall health.
Treg cells control the immune system through complex mechanisms that inhibit the activation and growth of other immune cells, particularly effector T-cells. They carry out these regulatory functions through direct cell-to-cell interactions and by secreting anti-inflammatory cytokines like IL-10 and TGF-β. Together, these processes help maintain immune balance, prevent excessive immune responses, and are essential for avoiding autoimmune reactions and supporting immune tolerance.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Horizon, Viela Deal; Concert Schizo Drug Flop; Gilead Deals with Arcus and Gritstone
- Immunocore’s Kimmtrak; Samsung Acquires Biogen’s Biosimilar Unit; Novavax’s COVID-19 Vaccin...
- Bayer’s AskBio Initiates Phase II GenePHIT Trial; FDA Approves Merck’s KEYTRUDA Plus Chemoradioth...
- Virus, the Cancer Therapy of the Future
- ZORYVE for Atopic Dermatitis Treatment: Is the Breakthrough We’ve Been Waiting for?
6 Treg Cell-based Therapies Gaining Traction
Treg therapies are being explored as treatments for several autoimmune disorders, such as multiple sclerosis, rheumatoid arthritis, and type 1 diabetes, by restoring immune tolerance. These therapies can also help prevent the immune system from attacking transplanted organs or tissues, potentially reducing the reliance on long-term immunosuppressive medications. Additionally, Treg therapies may offer benefits for inflammatory conditions like inflammatory bowel disease and psoriasis by helping to reduce chronic inflammation.
In addition to autoimmune disorders, Treg cell-based therapies are being investigated for cancer treatment. Tumors often manipulate Tregs to avoid immune detection, but by modifying these cells, it is possible to strengthen the immune response against tumors. Approaches include expanding Tregs outside the body and reinfusing them to increase their numbers, or altering them to more effectively target tumor-associated antigens. These methods have the potential to enhance the effectiveness of current immunotherapies and provide new options for treating cancers that are resistant to traditional therapies.
Currently over 50 companies are actively working with their lead assets to improve the therapeutic space, as per DelveInsight’s Treg Cell-based Therapies Pipeline Report. Let’s have a look at the 6 most promising Treg cell-based therapies that would revolutionize the treatment landscape in the coming years.
Orca Bio’s Orca-T
Orca-T is an experimental allogeneic cellular therapy that uses high-precision infusions containing regulatory T-cells, conventional T-cells, and CD34+ stem cells sourced from the peripheral blood of matched donors, either related or unrelated. It has been granted Regenerative Medicine Advanced Therapy (RMAT) designation by the FDA and is being investigated as a treatment for various hematologic malignancies. Currently, Orca-T is in Phase III of its development, targeting conditions such as acute myeloid leukemia, myelodysplastic syndromes, acute lymphoblastic leukemia, and mixed phenotype acute leukemia.
In December 2024, Orca Bio presented results from a three-year follow-up analysis of Orca-T combined with single-agent tacrolimus (TAC) in patients with acute myeloid leukemia, acute lymphoblastic leukemia, and high-risk myelodysplastic syndrome. The analysis showed promising overall survival (OS) rates and demonstrated improvement compared to a historical cohort of patients who received allogeneic stem cell transplant (alloHSCT) with post-transplant cyclophosphamide (PTCy)-based graft-versus-host disease (GvHD) prophylaxis.
This analysis was part of a multicenter Phase Ib clinical trial and involved 77 patients who received Orca-T with TAC. The outcomes were compared with 293 patients from a CIBMTR literature-based cohort who received alloHSCT with PTCy-based GvHD prophylaxis. All patients in both groups underwent myeloablative conditioning (MAC) and used a related or unrelated fully matched donor. The patient subsets were selected based on disease type, disease status, and conditioning regimens that closely aligned with those in the ongoing pivotal Phase III clinical trial of Orca-T.
The analysis covered patients with a median follow-up of 33 months (range 5-54 months) for the Orca-T Phase Ib group and 24 months (range 0-53 months) for the PTCy cohort. Further analysis suggested that Orca-T may reduce non-relapse mortality (NRM) and increase relapse-free survival (RFS) compared to PTCy. At one year, NRM was 1.4% in the Orca-T group, compared to 7.4% in the PTCy cohort, while the RFS rate at one year was 83% for Orca-T patients versus 71% for those receiving PTCy.
The results also indicated that age had no significant effect on OS at one year. In the Orca-T group, OS was 95% (88%-100%) for patients under 50 and 97% (92%-100%) for those over 50. In the PTCy group, OS was 86% (81%-92%) for patients under 50 and 77% (70%-85%) for those over 50. Across all patients in the Phase Ib trial, Orca-T was reliably manufactured and delivered with vein-to-vein times of 72 hours or less across the U.S.
Nektar Therapeutics’ NKTR-358
NKTR-358 is a groundbreaking, first-in-class stimulator of regulatory T (Treg) cells, aimed at correcting the immune system imbalance associated with autoimmune diseases and chronic inflammatory conditions. It functions by targeting the IL-2 receptor complex, selectively promoting the growth of Treg cells while avoiding activation of cytotoxic CD8+ T and CD4+ T cells, which contribute to autoimmune diseases. By stimulating Tregs, NKTR-358 helps suppress harmful disease-causing T cells and restores the body’s self-tolerance. The drug is currently being tested in a Phase II clinical trial for treating patients with alopecia areata and atopic dermatitis.
Quell Therapeutics’ QEL-001
QEL-001 is an innovative, first-in-class antigen-specific CAR-Treg cell therapy developed using Quell’s distinctive multi-modular engineered Treg platform. It incorporates three proprietary components: a chimeric antigen receptor (CAR) for targeted tissue engagement, the Foxp3 phenotype lock module, and a safety switch. The QEL-001 CAR is designed to target HLA-A2, directing the CAR-Treg activity to the site of the transplanted organ in patients with HLA-A2 mismatch liver transplants. The drug is currently being tested in a Phase I/II clinical trial for liver transplant patients.
In June 2024, Quell Therapeutics announced that it is progressing QEL-001, its autologous engineered CAR-Treg cell therapy, into the efficacy cohort of the LIBERATE Phase I/II trial for liver transplant patients. This move comes after the successful completion of dosing in the initial safety cohort (n=3) and approval from the trial’s independent Data Safety and Monitoring Board (DSMB) following their review of the clinical data.
The progress in the LIBERATE study was shared at the American Transplant Congress in Philadelphia, PA, USA, by Prof. Alberto Sánchez-Fueyo, Professor of Hepatology and Academic Director of the Institute of Liver Studies at Kings College London, as well as co-founder of Quell.
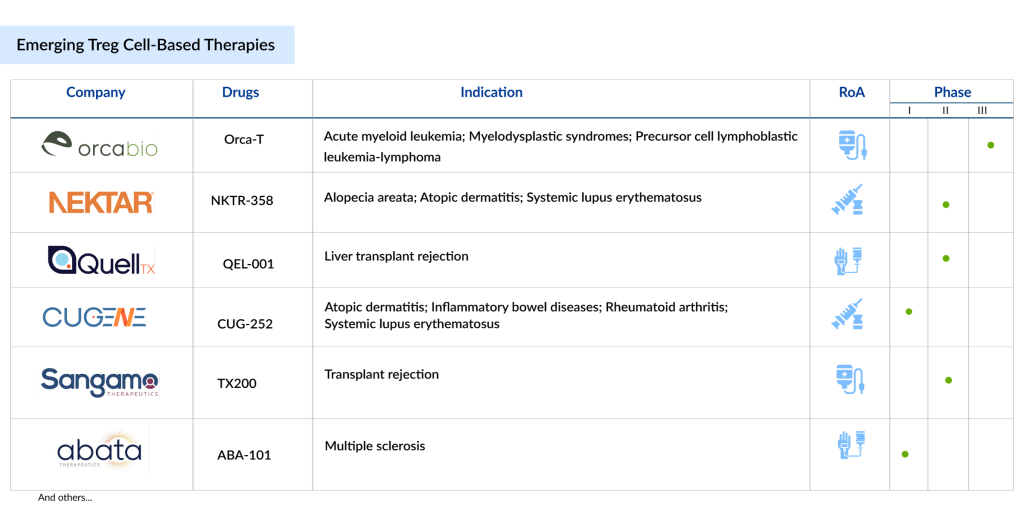
Sangamo Therapeutics’ TX200
TX200 is a therapeutic treatment made from the patient’s own (autologous) regulatory T cells (Tregs), which are genetically modified to express a chimeric antigen receptor (CAR) that targets the HLA-A2 protein found on a transplanted kidney. TX200 is being developed to prevent immune-mediated rejection in cases of HLA-A2 mismatched kidney transplantation from living donors.
For HLA-A2-negative patients, the process begins with a leukapheresis procedure to collect their white blood cells. The Treg cells are then isolated, genetically modified, and cryopreserved. Afterward, the patient undergoes surgery to receive a kidney from an HLA-A2-positive living donor. After recovery, the patient is administered the personalized TX200 cell therapy.
Once infused, TX200 cells are expected to migrate to the transplanted kidney and activate when they bind to the HLA-A2 antigen. By regulating the immune system, TX200 cells may help protect the transplant from rejection and potentially reduce or eliminate the need for long-term immunosuppressive treatments.
The drug is currently being evaluated in the phase I/II STEADFAST trial. The STEADFAST study is a Phase I/II, multicenter, open-label clinical trial designed to assess the safety and effectiveness of a single ascending dose of TX200 in preventing immune-mediated rejection in HLA-A2 mismatched kidney transplants from living donors.
The main goal of the study is to assess the safety and tolerability of TX200. Secondary objectives include evaluating TX200’s impact on acute graft-related outcomes, long-term safety, and its pharmacodynamic and pharmacokinetic effects.
Routine post-transplant biopsies will help detect engineered CAR-Tregs in the kidney, while adjustments to standard immunosuppressive therapy will be made at the investigator’s discretion.
Cugene’s CUG-252
CUG252 is a precisely designed, long-lasting IL-2 that selectively targets Tregs, promoting their expansion with high specificity and minimal toxicity in enhancing their function. By re-establishing immune balance and reducing autoreactivity, CUG252 shows great potential as a new treatment for a range of autoimmune and inflammatory disorders.
The drug is currently being evaluated under Phase I clinical trial for the treatment of patients with atopic dermatitis, inflammatory bowel diseases, rheumatoid arthritis, and systemic lupus erythematosus.
Abata Therapeutics’ ABA-101
ABA-101 is an autologous Treg cell therapy designed to directly address chronic, smoldering multiple sclerosis pathology and facilitate the repair of damaged myelin in the central nervous system (CNS) of multiple sclerosis patients. This therapy involves engineering a patient’s own Tregs to express a T cell receptor (TCR) that targets degraded myelin within the CNS. Due to their specificity for the degraded myelin, ABA-101 Tregs are expected to become active in the affected CNS tissues, where they can suppress harmful immune activity and help restore balance.
ABA-101 was developed for multiple sclerosis patients with progressive disease who show imaging evidence of ongoing, smoldering inflammatory injury in the CNS. In preclinical studies, ABA-101 demonstrated safety, targeted tissue-specific movement, persistence, and effective suppression of inflammation, supporting its potential as a therapeutic solution. Abata is currently conducting the first human clinical trial of ABA-101.
Future Outlook of Treg Cell-based Therapies
The future outlook of Treg-based therapies holds immense promise in the field of immunotherapy, particularly for autoimmune diseases, cancer, and transplant rejection. As research continues to unravel the intricate roles of Tregs in immune system modulation, advancements in genetic engineering and cell-based therapies are expected to enable more precise and targeted approaches.
The ability to manipulate Tregs to suppress unwanted immune responses while promoting immune tolerance opens up new avenues for treating diseases like type 1 diabetes, inflammatory bowel disease, and graft-versus-host disease. Additionally, Treg-based therapies could help enhance the efficacy of other immunotherapies, such as cancer immunotherapies, by fine-tuning immune responses in the tumor microenvironment.
As clinical trials progress, the scalability, safety, and efficacy of Treg cell therapies will likely improve, making them more accessible to a broader patient population. The integration of technologies like CRISPR and artificial intelligence for a better understanding of Treg function and optimization of cell manufacturing processes will accelerate their development.
Challenges such as the risk of over-suppressing immune responses and maintaining the long-term stability of modified Tregs must be addressed, but ongoing advancements in biomarker discovery and patient stratification are paving the way for more personalized and effective treatments. With continued progress, Treg-based therapies have the potential to revolutionize the treatment of various chronic and complex diseases, offering a transformative approach to immune modulation.

Downloads
Article in PDF
Recent Articles
- Biogen buys Nightstar; Pacira acquires Myoscience; Horizon Pharma announces pricing; STAT with Sl...
- Gilead Buys Out Rights to Cancer Therapy from Jounce; FDA Places Clinical Hold on Biogen’s Orelab...
- Immutep’ First-Line Treatment Positive Outcomes; Pfizer’s Once-Daily Oral GLP-1 Agonist Danuglipr...
- The Growing Burden Of Neurodegenerative Disorders
- FDA Fast Track Status to Kyverna’s KYV-101; Annovis’s Phase III Study for Buntanetap; Gilteritini...