AstraZeneca’s AKT Inhibitor TRUQAP Falls Short in Triple Negative Breast Cancer
Jun 25, 2024
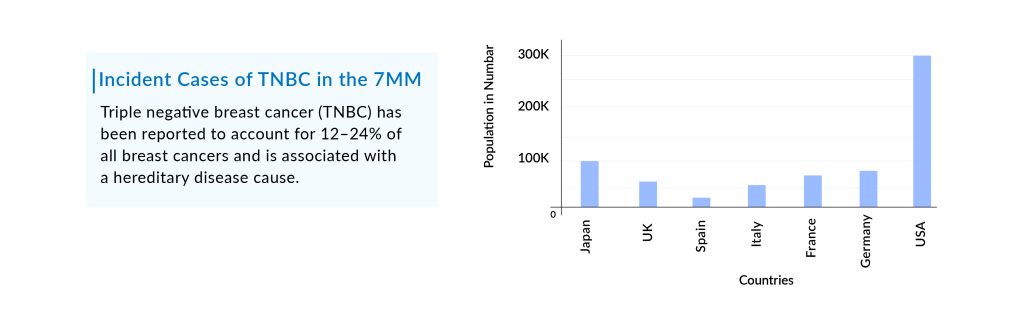
Triple-negative breast cancer (TNBC) constitutes about 10-15% of all breast cancer cases with nearly 44,000 incident cases in the United States in 2023, as per Delveinsight’s estimates. It is a rare but aggressive form of breast cancer. Treatment for TNBC is more limited than for other breast cancer types.
Current Landscape of TNBC Treatment
Treatment can be challenging for TNBC. Without receptors, triple-negative tumors do not have the proteins they need to respond to common breast cancer treatments like hormone therapy and targeted therapy used for HR+/HER2- breast cancer. In patients with localized TNBC, dose-dense doxorubicin-cyclophosphamide, and paclitaxel were the standard neoadjuvant chemotherapy backbone until 2021.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Idera’s drug; CRISPR increase risk; Juvenescence grabbed USD 50M; AstraZeneca, Lilly termin...
- Bayer Partners with DelSiTech to Develop; Amgen and Arrowhead Announce; Allergan, AstraZeneca’s d...
- Top 5 Cancers Creating Major Challenge To The Global Healthcare System
- Business Cocktail
- A New Star at SABCS 2025: BioNTech–BMS’s Pumitamig Redefines Next-Gen Immunotherapy
With the approval of neoadjuvant KEYTRUDA (pembrolizumab), chemotherapy with immunotherapy became the new standard of care for localized TNBC. The second most preferred form of treatment is PARP inhibitors, LYNPARZA (olaparib) and TALZENNA (talazoparib); their usage is preferred in patients with germline BRCA1/2 mutations. For patients with recurrent/refractory metastatic TNBC, Gilead’s TROP-2 antibody-drug conjugate, TRODELVY (sacituzumab govitecan-hziy) has emerged as an effective treatment option, especially in the second line of TNBC treatment.
TRUQAP breaking the winning streak of AstraZeneca
AstraZeneca is evaluating its AKT inhibitor in multiple cancer indications, leading to approval in one type of breast cancer. TRUQAP is the first AKT inhibitor to gain US FDA approval, however, it is encountering challenges in broadening its label. Approximately 35% of TNBC cases show alterations in the PIK3CA, AKT1, and PTEN proteins. Previously, in one of its Phase II PAKT study, the combination of TRUQAP and paclitaxel demonstrated prolonged PFS and OS vs. placebo plus paclitaxel as frontline therapy in patients with advanced TNBC. However, the drug failed in the Phase III CAPItello-290 study in TNBC which broke the company’s winning streak (with IMFINZI, CALQUENCE, and TAGRISSO). CAPItello-290 was designed as a global, double-blind, randomized trial to further evaluate the efficacy and safety of TRUQAP plus paclitaxel vs. placebo plus paclitaxel as frontline treatment in 923 patients with locally advanced inoperable or metastatic TNBC.
The company has not yet offered any specific data but it has been revealed that the trial had failed to meet its main goal, i.e., to improve overall survival. However, on the bright side, the safety profile of both drugs was consistent, with no new safety signals detected.
“Despite modest advances, triple-negative breast cancer remains one of the most challenging forms of disease to treat due to the lack of known actionable biomarker targets, and chemotherapy-based regimens continue to be the mainstay of treatment. While the CAPItello-290 trial results have not shown what we hoped, they provide important information to further understand this aggressive form of breast cancer where patients are in urgent need of new treatments.”
Principal Investigator of the trial
Despite the miss, what is AstraZeneca’s development path for TRUQAP?
The company has been trying to expand TRUQAP’s patient pool by testing it in other types of breast cancer and other settings of care. CAPItello-290 was a part of this push, alongside the Phase III CAPItello-292 study, which is testing the AKT inhibitor with FASLODEX and palbociclib as a first-line triplet regimen in patients with locally, inoperable or metastatic HR+/HER2- breast cancer. In June 2024, TRUQAP+FASLODEX was approved in the EU for the treatment of adult patients with ER+/HER2- locally advanced or metastatic breast cancer with one or more PIK3CA, AKT1, or PTEN-alterations following recurrence or progression on or after an endocrine-based regimen. The approval was based on results from the CAPItello-291 Phase III trial. AstraZeneca is also investigating TRUQAP in prostate cancer, for which it is running the CAPItello-280 and CAPItello-281 trials. TRUQAP reported sales of USD 50 million in the first quarter of 2024, and USD 6 million in sales during the fourth quarter of 2023. The recent approval in the EU is expected to enhance the drug’s sales.
Other companies that are engaged in the development of the treatment for TNBC are Gilead Sciences, Merck, Roche, Daiichi Sankyo, G1 Therapeutics, and others have been listed below:

Downloads
Article in PDF
Recent Articles
- NATCO Pharma surges; AstraZeneca falls; US patent office rules
- AZ shows positive results for its COVID-19 vaccine; Synairgen offers a new approach to address CO...
- Notizia
- Ethicon’s ETHIZIA Hemostatic Sealing Patch; FDA Approves Medtronic’s Minimally Invasive Device to...
- The Most Promising COVID-19 Vaccine Candidates In The Pipeline To Watch Out For



