Idorsia’s Tryvio Success in Hypertension Treatment After J&J ‘Blunder’?
Apr 08, 2024
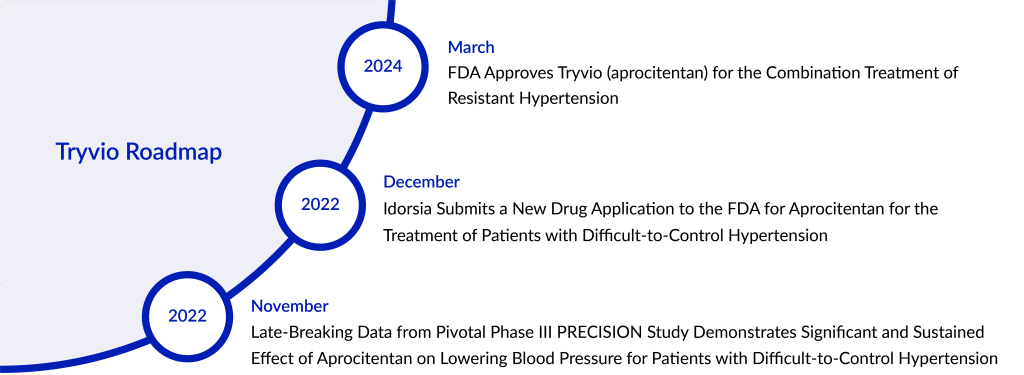
After Johnson & Johnson reversed its decision last year regarding the acquisition of Tryvio (aprocitentan) in 2017, the original manufacturer of the hypertension treatment drug, Idorsia Pharmaceuticals has successfully guided the drug through the FDA approval process.
The company stated that Tryvio, its endothelin receptor antagonist, has received FDA approval. Tryvio is an oral hypertension medication designed to be taken once daily in conjunction with other antihypertensive medications. It is intended for hypertension treatment in adults who haven’t achieved sufficient control with other hypertension medications, aiming to lower blood pressure levels.
It has been close to four decades since a novel therapeutic mechanism led to FDA approval for an oral medication for hypertension treatment. Tryvio marks the first FDA-approved drug designed to address hypertension by focusing on the endothelin pathway. Current therapies function through various methods, such as controlling salt and water balance, decreasing extracellular calcium entering cells, and blocking the renin-angiotensin-aldosterone system.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Treatment-resistant Hypertension: Insights into the Challenges and Solutions
- The Rise of Energy Drinks: Power in a Can or a Health Hazard?
- One of the leading causes of blindness, and a sight-threatening disease, Uveitis, now has novel t...
- Leman Micro Devices’ e-Checkup system; Calon Cardio-Technology & Leviticus-Cardio announced ...
- Ethicon’s ETHIZIA Hemostatic Sealing Patch; FDA Approves Medtronic’s Minimally Invasive Device to...
Jean-Paul Clozel, MD and Chief Executive Officer of Idorsia, remarked that there exists a significant public health concern in the United States with millions of individuals having poorly managed blood pressure despite available treatments. This situation often results in a high occurrence of cardiovascular and cerebrovascular events. To confront this challenge, Idorsia has developed aprocitentan, an endothelin receptor antagonist designed specifically for these patients. Idorsia embarked on an extensive clinical program involving patients who remained hypertensive despite being on a minimum of three drugs at their optimal doses, and in some cases, even four, five, or six antihypertensives. Dr. Clozel expressed great pride in the Idorsia team and joy in providing physicians with a new treatment option for patients struggling with uncontrolled blood pressure.
Idorsia supported Tryvio’s application for regulatory approval by providing data from the Phase III PRECISION hypertension clinical trial study. The effectiveness of Tryvio was assessed in a complex Phase III multicenter study involving adults whose systolic blood pressure (SBP) was at least 140 mmHg and who were taking a minimum of three antihypertensive medications. This study consisted of multiple parts, starting with a placebo phase followed by three distinct stages, detailed below. Before the placebo phase, all participants were transitioned to a standard background treatment plan for hypertension, which included an angiotensin receptor blocker, a calcium channel blocker, and a diuretic, maintained throughout the entire study. Patients already using beta-blockers continued this treatment throughout the study period.
After a 4-week period where patients received a placebo, 730 individuals were randomly assigned in equal numbers to receive aprocitentan at doses of 12.5 mg, 25 mg, or a placebo once a day for the initial 4-week double-blind treatment phase (part 1). Following this phase, all participants progressed to a single-blind treatment period (part 2), where they were given 25 mg of aprocitentan once daily for 32 weeks. After 32 weeks, patients were again randomized to receive either 25 mg of aprocitentan or a placebo once daily during a 12-week double-blind withdrawal period (part 3).
The main focus of effectiveness was the difference in seated systolic blood pressure (SiSBP) from the beginning to Week 4 in the initial phase, assessed at its lowest point using an unattended automated office blood pressure (uAOBP) monitor. Another important measure was the change in SiSBP at its lowest point using uAOBP from Week 36 (before random assignment to 25 mg aprocitentan or placebo in the final phase) to Week 40.
The patients, on average, were 62 years old (ranging from 24 to 84 years), with 60% being male. The majority were White (83%), followed by African American (11%) or Asian (5%). Around 10% of the patients identified as Hispanic. The average body mass index (BMI) was 34 kg/m2 (ranging from 18 to 64 kg/m2).
At the beginning of the study, 19% of the patients had an estimated glomerular filtration rate (eGFR) of 30–59 mL/min/1.73 m2, and 3% had an eGFR of 15–29 mL/min/1.73 m2. Additionally, 24% had a urine albumin-to-creatinine ratio (UACR) of 30–300 mg/g, while 13% had a UACR greater than 300 mg/g.
About 54% of the patients had a history of diabetes mellitus, 31% had ischemic heart disease, and 20% had congestive heart failure. Finally, at the study’s outset, 63% of patients reported taking four or more antihypertensive medications.
Alberto Gimona, MD and Head of Global Clinical Development of Idorsia stated: “In creating PRECISION, we made sure to include patients who face the highest risks from the severe complications of hypertension. Furthermore, while all participants were required to be taking three antihypertensive medications to enroll, 63 percent were actually on four or more. This made the study a true representation of the real-world population of patients whose blood pressure remains uncontrolled despite other treatments. Tryvio has shown a clear and consistent impact across all measurements of blood pressure and among important subgroups. Consequently, Tryvio offers promise as a new, effective, and well-tolerated option for patients with inadequately controlled hypertension.”
Tryvio 12.5 mg showed significant superiority over a placebo in reducing sitting systolic blood pressure (SiSBP) by Week 4 (part 1) of the trial. This beneficial effect was also observed consistently for sitting diastolic blood pressure (SiDBP). The hypertension clinical trial’s third phase further confirmed the long-lasting blood pressure-lowering impact of Tryvio. During this phase, patients initially on aprocitentan were randomized again, either to placebo or to a continued dosage of 25 mg aprocitentan, after an initial treatment period with 25 mg for all patients. Among those re-randomized to placebo, the average SiSBP increased, contrasting with the group re-randomized to 25 mg aprocitentan, where the mean effect on SiSBP was upheld and statistically superior to placebo by Week 40. This consistent treatment effect also extended to SiDBP.
The majority of the blood pressure reduction occurred within the initial two weeks of using Tryvio. However, it’s important to note that Tryvio is not approved for utilization at a 25 mg dosage. When examining the effectiveness of aprocitentan at the 25 mg level, specifically regarding the primary outcome of change in sitting systolic blood pressure (SiSBP) from the beginning to Week 4 in the first part of the study, it was found to be comparable to the 12.5 mg dose. Therefore, the approved dose for aprocitentan is 12.5 mg.
The consistent lowering of blood pressure with Tryvio was observed across various subgroups, including those defined by age, gender, race, body mass index (BMI), baseline estimated glomerular filtration rate (eGFR), baseline urinary albumin-to-creatinine ratio (UACR), history of diabetes, and different methods of blood pressure measurement (office and ambulatory measurements).
The most commonly documented negative effects of Tryvio during the 4-week double-blind placebo-controlled treatment phase (part 1) of the PRECISION study were swelling/fluid buildup and low red blood cell count. Within this initial 4-week period of double-blind placebo-controlled treatment (part 1), 0.8% of patients encountered a negative reaction of heightened sensitivity (such as rash, skin redness, allergic swelling) while on Tryvio, compared to zero reports among patients given a placebo. A single patient experienced an allergic skin inflammation requiring hospital care while taking aprocitentan 25 mg. Individuals who are hypersensitive to aprocitentan or any of its ingredients should avoid using Tryvio. The usage of Tryvio is not recommended during pregnancy.
Reducing blood pressure decreases the chances of fatal and non-fatal cardiovascular incidents, especially strokes and heart attacks. Such advantages have been observed in controlled studies of different types of antihypertensive medications. However, there haven’t been any controlled trials showing a decrease in the risk of these events with Tryvio.
Tosh Butt, President and General Manager of Idorsia US remarked on the approval of Tryvio in the US, stating it as a significant achievement for Idorsia. Describing Tryvio as an innovative hypertension medication with a distinctive approach to addressing systemic hypertension, Butt highlighted Idorsia’s expertise and long-standing involvement in the field of endothelin receptor antagonism. He expressed eagerness to introduce physicians and patients to this new medication, which operates in a different pathway for managing uncontrolled hypertension and offers added blood pressure control. Recognizing the substantial resources needed to reach the entire prescribing community, Butt mentioned that Idorsia will be carefully developing the Tryvio launch strategy over the next few months, with plans to make Tryvio available in the latter half of 2024.
At one stage, Johnson & Johnson had faith in the drug, as evidenced by its $230 million acquisition of the hypertension medication in 2017. Initially, J&J was slated to market Tryvio upon potential approval, with Idorsia receiving royalties. However, in September of last year, the pharmaceutical giant returned the drug to its original manufacturer in a deal valued at up to 306 million Swiss francs ($343 million). This arrangement included specific terms allowing J&J to receive 30% of any revenue generated from licensing or divesting the drug, as well as 10% of proceeds from deals involving any Idorsia products post Tryvio’s FDA approval.
Tryvio carries a black box warning concerning embryo-fetal toxicity, highlighting the potential for significant birth defects if used during pregnancy. It is advised that patients who are pregnant or actively attempting to conceive should not take Tryvio.
Tryvio is accessible solely via a limited program known as the Tryvio REMS due to the potential danger of harm to embryos and fetuses. Healthcare providers need to undergo certification through the Tryvio REMS by enrolling in and finishing the required training. Pharmacies that provide Tryvio must also hold certification under the Tryvio REMS.

Downloads
Article in PDF
Recent Articles
- The Question That Remains Unanswered: What Might Be Causing Alzheimer’s?
- Treatment-resistant Hypertension: Insights into the Challenges and Solutions
- Regeneron’s Odronextamab BLA; Novo Nordisk’s Cardior Pharmaceuticals Acquisition; Novartis’ Fabha...
- Immunocore’s Kimmtrak; Samsung Acquires Biogen’s Biosimilar Unit; Novavax’s COVID-19 Vaccin...
- World Hypertension day