Comprehensive Assessment of the Leading Companies in the Vaccines Market
Jul 08, 2022
Table of Contents
Millions of lives are saved annually all because of vaccination, which is a success story in global health and development. More than 20 life-threatening diseases can now be prevented with vaccines, allowing individuals of all ages to live longer, healthier lives. Vaccines prevent 3.5–5 million deaths yearly from diseases like measles, diphtheria, tetanus, pertussis, and influenza.
Immunization is an unquestionable human right and an essential part of primary healthcare. It’s also among the finest investments in health that money can buy. Infectious diseases outbreaks can be prevented and controlled with the use of vaccines. They support the security of the world’s health and will be a crucial weapon in the fight against antibiotic resistance.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- GE Healthcare-Optellum’s collaboration; Ocugen’s Covid-19 vaccine trial; Blueprint to acqui...
- MedTech Wrap Up
- Abingworth & Alebund’s Finacial Closing; Pfizer/BioNTech COVID-19 Vaccine Expanded Use...
- While AZ resumes its COVID-19 vaccine trial in the UK; Merck and Gilead are busy making sizeable ...
- Bayer’s CKD Drug; Enochian’s RNA Hijack for HBV; Novo Nordisk’s to get Prothena’...
Evolution of Vaccines
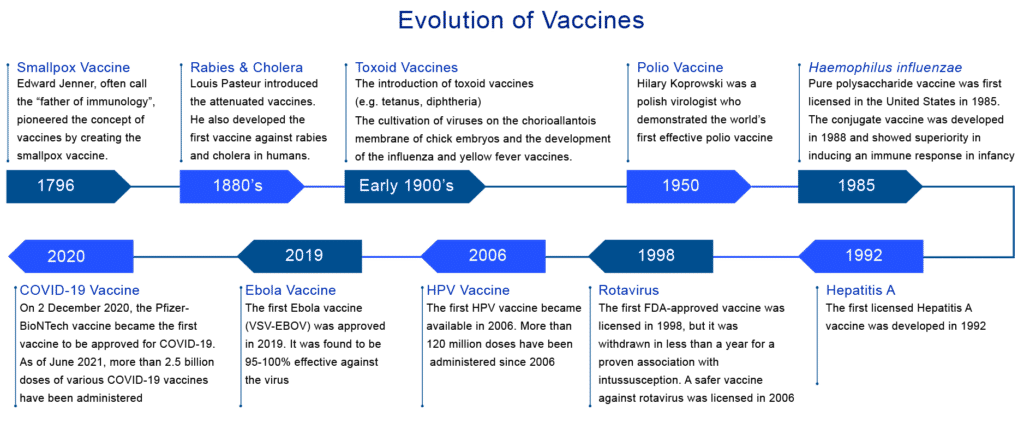
The beginning of the vaccine evolution can be traced back to the late 18th century when in 1796, Dr. Edward Jenner inoculated a 13 year-old-boy with the vaccinia virus (cowpox) and demonstrated immunity to smallpox. In 1798, the first smallpox vaccine was developed. In 1897 and 1904, Louis Pasteur’s experiments escalated the development of live attenuated cholera and inactivated anthrax vaccines, respectively. By the late 19th century, the plague vaccine was also invented. From 1890 to 1950, the bacterial vaccine’s development proliferated, including the Bacillus-–Calmette Guerin (BCG) vaccination currently in use.In 1923, Alexander Glenny inactivated tetanus toxin with formaldehyde, and a similar method was used to develop a vaccine against diphtheria in 1926. In 1948, a whole-cell pertussis vaccine was licensed for use. The advent of viral tissue culture between 1950 and 1985 led to the development of the Salk (inactivated vaccine) polio vaccine and the Sabin (live attenuated oral) polio vaccine. However, over the past two decades, the application of molecular technology has been considerably seen in the development of technologically advanced vaccines.
Live vs. Non-live Vaccines
The demarcation between live and non-live vaccines is essential. Live vaccines may replicate uncontrollably in immune-compromised individuals (for example, individuals with HIV infection, children with some primary immunodeficiencies, or those receiving immunosuppressive drugs), which may severely restrict their applicability in such patients. By contrast, non-live vaccines offer a viable option for immunocompromised individuals.
Live vaccines are developed in such a way that in an immunocompetent host, they replicate to induce a strong immune response, but not enough to result in disease manifestations (for example, the vaccines for oral polio vaccine, measles, mumps, rubella, and rotavirus, the Mycobacterium bovis bacillus Calmette–Guérin (BCG) vaccine for TB and live attenuated influenza vaccine). There is a trade-off between enough replication of the vaccine pathogen to induce a strong immune response and sufficient attenuation of the pathogen to avoid symptomatic disease.
For this reason, some safe, live attenuated vaccines require multiple doses and induce relatively short-lived immunity (for example, the live attenuated typhoid vaccine, Ty21a), and other live attenuated vaccines may induce some mild disease (for example, about 5% of children will develop a rash and up to 15% fever after measles vaccination).
For non-live vaccines, the antigenic component can comprise killed whole organisms (for example, inactivated polio vaccine and whole-cell pertussis vaccine), purified proteins from the organism (for example, acellular pertussis vaccine), polysaccharides (for example, the pneumococcal vaccine against S. pneumoniae), and recombinant proteins (for example, hepatitis B virus (HBV) vaccine). Toxoid vaccines are formaldehyde-inactivated protein toxins that have been purified from the pathogen (for example, for diphtheria and tetanus). Non-live vaccines are often combined with an adjuvant to improve their immunogenicity. Currently, only a few adjuvants are used routinely in licensed vaccines. A few examples of these adjuvants include alum, MF59, AS04, and AS01, among others.
Generation and Types of Vaccines
Vaccines can be broadly classified by how the antigens that generate a specific immune response against the disease-causing organism are prepared. Based on the development method, the vaccines are subcategorized under different generations.
First Generation Vaccines: Attenuated pathogens, full organisms, or inactivated bacterial toxins, which are effectively immunogenic, are used in making these vaccines and are considered first-generation vaccines.
Second Generation Vaccines: The basis of the second-generation vaccines were subunit elements, recombinant or synthetic proteins, nonprotein antigens, and expressed bacterial immunogens or viruses, which include numerous molecules and epitopes of different species and strains of pathogens. These vaccines were developed to overcome the possibility of recurrence in the virulence of the live attenuated vaccines.
Third Generation Vaccines: Genetic vaccines are categorized as third-generation vaccines in which a plasmid comprising a gene encoding the antigen is administered. Different names have been given for these kinds of vaccines, such as DNA, RNA, and plasmid.
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Factors Driving the Vaccines Market
The consistent focus on childhood immunization programs
One of the prominent factors that led to the growing popularity and high uptake of vaccines across the globe was the extensive focus on carrying out childhood immunization programs to reduce childhood morbidity and mortality rates across the globe. In 1974, the World Health Organization (WHO) created the Expanded Programme on Immunization (EPI), a worldwide effort that helped countries increase immunization coverage of basic childhood vaccines—measles, diphtheria, polio, pertussis, tetanus, and tuberculosis—using the third dose of diphtheria, tetanus, and pertussis (DTP3) as a measure of progress.
Furthermore, as per the WHO, since 1990, over 1.1 billion children have been immunized against vaccine-preventable diseases, thereby considerably reducing child mortality by half and saving 4–5 million lives a year. Additionally, in 2021, 15 million children in the Eastern Mediterranean Region of the WHO were vaccinated. Therefore, the continuous global efforts by different international and national organizations across the globe to combat childhood morbidity and mortality due to preventable diseases is a major driving factor for the growing demand for vaccines.
Growing incidence of cancers
Another aspect stimulating the growth of the vaccine market is the rising incidence of cancers and the growing efforts to provide preventive immunity against certain cancers. As per the WHO (2022), cervical cancer is the fourth most common cancer among women globally, accounting for an estimated 604,000 new cases and 342,000 deaths in 2020. The source further stated that nearly 90% of the new cases and deaths in 2020 across the globe occurred in low-and middle-income countries. Currently, preventive cancer vaccines are available for human papillomavirus and Hepatitis B virus. The human papillomavirus stays in the body for too long and can develop cervical, vaginal, and anal cancer.
Similarly, the hepatitis B virus can cause liver cancer. The preventive vaccine against HPV is said to be administered between the ages of 11–12 years before the commencement of sexual activity to avoid HPV exposure. Some of the therapeutic vaccines approved by the FDA that are available in the vaccines market are BCG live vaccine for early-stage bladder cancer, siplucel-T for prostate cancer, and talimogene laherparrepvec for melanoma.
The exigency created by the COVID-19 pandemic
The urgency created by spreading the SARS CoV-2 virus across the globe paved the way for one of the deadliest pandemics of the 21st century. Due to the novel nature, high transmissibility, and viral infectivity of this virus, there was a dire need for novel preventive and therapeutic measures as well. According to the WHO, as of June 9, 2022, there have been 531,550,610 confirmed cases of COVID-19, including 6,302,982 deaths. As of June 6, 2022, 11,854,673,610 vaccine doses have been administered. This has become possible due to extensive research and development in developing prophylactic vaccines to immunize people against the highly infectious SARS CoV-2 virus.
The COVID-19 vaccines started gaining regulatory approvals across the world, with the Comirnaty COVID-19 messenger mRNA vaccine by Pfizer/BioNTech receiving the first emergency use authorization from the WHO. After that, numerous COVID-19 vaccines have gained approval across the globe, such as Ad26.COV2.S by Janssen, Vaxzevria by Oxford/AstraZeneca, Spikevax by Moderna, and Coronavac by Sinovac, among others.
Technological advancements in vaccine development
Emerging non-viral vaccine technologies such as nanoparticle vaccines, DNA or RNA vaccines, viral-like particles, and rational vaccine design have paved the way for inventive approaches to overcome the challenges presented by conventional vaccine technologies. Besides advances in vaccine technology, including structural biology in identifying structures of antibodies and their targets may significantly improve the designed immunogens.
Some Prominent Players in the Vaccines Market
The vaccines market is highly competitive, with numerous international and regional players in the vaccines market. The leading vaccines companies such as GlaxoSmithKline, Serum Institute of India, Bharat Biotech, AstraZeneca, Bavarian Nordic A/S, BioNTech, Sanofi, Merck & Co., Inc, Pfizer Inc, Moderna, Mitsubishi Chemical Holdings Corporation, EMERGENT, CSL Limited, Janssen Global Services, VBI Vaccines Inc., Valneva SE., SEQIRUS, Novavax, and several others are currently working in the vaccines market. Among them, the top vaccine companies dominating the vaccines market are

GSK
GlaxoSmithKline, one of the world’s largest vaccines companies with an extensive focus on delivering vaccines that help protect people at all stages of life. It is a publicly-listed company, and it is headquartered in the United Kingdom. Their R&D focuses on developing vaccines against infectious diseases that aims to address both strong market potential and high medical need. The company has an extensive vaccine portfolio and offers vaccines for infectious diseases such as smallpox, meningitis, pneumococcal disease, whooping cough, hepatitis, influenza, and others. GSK has more than 20 vaccines as part of its vaccine business portfolio, and they are supplied in more than 160 countries.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sanofi
Sanofi is one of the leading companies involved in vaccine development. It is a publicly-listed company and is headquartered in France. The company has an extensive network of operations across the globe, with 20 R&D sites and 70 production sites. The medicines and vaccines developed by Sanofi are available across 170 countries. The company’s key focus is to provide potentially life-changing treatments and the protection of life-saving vaccines to millions of people across the globe and affordable access to life-saving vaccines in some of the world’s poorest countries.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Merck & Co Inc
Merck & Co, Inc was established in 1891 and headquartered in New Jersey; the United States is a global research-driven pharmaceutical company that focuses on developing prescription medicines, biological therapies, vaccines, and animal health products. The company has two major operating segments- pharmaceutical and animal health segments. The pharmaceutical segment encompasses vaccines as a product type. The company has a strong footprint across the globe. The company has its major operations in the United States, Europe, Japan, Asia-Pacific, and Latin America.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pfizer Inc.
Pfizer Inc. is a research-based, global biopharmaceutical company. The company is majorly focused on the discovery, development, manufacture, marketing, sale, and distribution of biopharmaceutical products worldwide. It is a publicly-listed company and is headquartered in the United States. The company generates most of its revenue from manufacturing and selling biopharmaceutical products. On August 23, 2021, FDA announced the first approval of a COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 vaccine and will now be marketed as Comirnaty for preventing COVID-19 in individuals 16 years of age and older.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Serum Institute of India
Serum Institute of India Pvt. Ltd. is considered the world’s largest vaccine manufacturer by the number of doses produced and sold globally (more than 1.5 billion doses), which includes Polio vaccine, Diphtheria, Tetanus, Pertussis, Hib, BCG, r-Hepatitis B, Measles, Mumps, Rubella as well as Pneumococcal and COVID-19 vaccines. Vaccines manufactured by the Serum Institute are accredited by the World Health Organization, Geneva, and are being used in around 170 countries across the globe in their national immunization programs, saving millions of lives worldwide. The company was founded in 1966. It is a private company headquartered in Pune, India.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recent Developments in the Vaccines Market
- In May 2022, the World Health Organization (WHO) issued an emergency use listing (EUL) to a vaccine manufactured by CanSino Biologics, China-CONVIDECIA, thereby adding another vaccine to the growing portfolio of vaccines validated by WHO for the prevention of COVID-19 caused by SARS-CoV-2.
- In October 2021, the World Health Organization recommended the first malaria vaccine for children- Mosquirix by GSK, which was considered a breakthrough by the UN agency as a “historic moment.”
- In July 2021, Merck received regulatory approval from the FDA for their VAXNEUVANCE™ (Pneumococcal 15-valent Conjugate Vaccine) developed intending to prevent invasive pneumococcal disease in adults 18 years and older caused by 15 serotypes.
Conclusion
According to DelveInsight analysis, the vaccines market is expected to witness significant progress in growth. The global vaccine market size will grow at a significant CAGR to surpass USD 116 billion mark by 2027.
This can be attributed to various factors such as the increasing product demand due to the rising prevalence of various infectious diseases, increasing incidence of cancers, along with the outbreak of the COVID-19 pandemic resulting in the surge in demand for prophylactic as well as therapeutic vaccines in the vaccines market.
Furthermore, technological advancements have led to the transformation of vaccine technology, including the development of new vaccine delivery systems (e.g., DNA vaccines, viral vectors, topical formulations, and plant vaccines), new adjuvants, the development of more effective tuberculosis vaccines, and vaccines against cytomegalovirus (CMV), herpes simplex virus (HSV), respiratory syncytial virus (RSV), HIV and schistosomiasis among others and the growing interest in developing therapeutic vaccines for various diseases such as tuberculosis, autoimmune diseases, and substance abuse.
Additionally, the extensive focus on adjuvant research is another aspect that may help develop vaccines as numerous benefits of adjuvanted vaccines are reported across various research. For instance, adjuvants enhance the efficacy of a vaccine using less antigen, which can be crucial in cases where the antigen may be in short supply or costly, which may expand the number of vaccine doses available. Also, adjuvanted vaccines can elicit more robust immune responses, reducing or eliminating the need for booster vaccinations, ultimately simplifying immunization schedules.

Moreover, the implications posed by the COVID-19 pandemic negatively affected the vaccines market growth during the initial phase of the COVID-19 pandemic. The restrictions imposed on the global scale to break the chain of infection spread led to disruptions in product manufacturing, supply chains, as well as procurement of raw materials in the vaccines market. This massively affected childhood immunization programs across the globe. As per the data provided by UNICEF, in 2020, 23 million children did not receive basic childhood vaccines through routine health services, which was the highest number since 2009 and about 3.7 million more compared to 2019. However, the onset of the COVID-19 pandemic also presented a massive opportunity for vaccine manufacturers to meet the exigent need for COVID-19 vaccines, which led to the research, development, and manufacture of numerous COVID-19 vaccines across the world. As the impact of the COVID-19 pandemic started to subside, the overall vaccines market is on the path to recovery as product demand starts returning to pre-COVID-levels due to the resumption of activities across virtually all domains, including healthcare, thereby presenting a conducive growth environment for vaccines manufacture and supply in the coming years.
Downloads
Article in PDF
Recent Articles
- What is Driving the Pertussis Treatment Market Forward?
- Widespread Usage of HPV Vaccine Reduces Cervical Cancers and Precancers
- World Immunization Week
- Biosense Webster’s HELIOSTAR Radiofrequency Balloon Ablation Catheter; Philips’s ClarifEye Augmen...
- FTA for Gannex’s ASC4; Disappointment for Incyte’s Ruxolitinib; Historic win for Pfizer, BioNTech...