Revolutionizing the Food Allergy Treatment: The Impact of Xolair’s Approval
Mar 01, 2024
Individuals suffering from food allergies now have access to a medication that can potentially avert serious consequences. This medication, which has been available for twenty years, has received approval from the FDA. Xolair (omalizumab) by Roche and Novartis is the first food allergy treatment sanctioned to diminish allergic responses stemming from unintentional consumption of specific foods. It is authorized for individuals aged one year and above who have particular allergies.
The food allergy treatment is intended for individuals with IgE-mediated food allergies, a category encompassing 160 different foods, with the most prevalent being peanuts, milk, eggs, wheat, soy, and tree nuts. Xolair is not formulated to enable patients to consume these foods without restrictions. Instead, it assists them in preventing severe reactions, such as anaphylaxis—a potentially life-threatening situation that can occur suddenly when the immune system releases chemicals, potentially inducing shock in the body.
Levi Garraway, M.D., Ph.D., Genentech’s chief medical officer and head of Global Product Development, stated that Xolair presents patients and families with a significant new treatment choice. This option could potentially redefine the approach to managing food allergies, aiming to decrease the often severe allergic reactions triggered by exposure to food allergens. Dr. Garraway highlighted that the recent approval marks a progression from two decades of patient feedback, with a proven track record of effectiveness and safety established since Xolair’s initial authorization for allergic asthma. The company is eager to introduce this treatment to the food allergy community, fulfilling a long-awaited advancement in care.
Downloads
Article in PDF
Recent Articles
- Sygnis AG and ECACC Collaboration; BioNTech and Genentech Partnership; Partnership between Zydus ...
- Biodesix collaborates with MRM; Avoiding flu with an antibody; Soliris clears trial
- Sanofi acquires Principia; FDA’s Nod to Roche’s Enspryng; BMS, Dragonfly’s Lice...
- Spinal Muscular Atrophy Market is expected to augment at a CAGR of 10.42%
- Acquisition of Stemline; Roche’s Elecsys Anti-SARS-CoV-2 antibody test; Newron’s abandonment of S...
According to Genentech, a subsidiary of Roche, the United States has 3.4 million children with food allergies, with over 40% of them experiencing a severe reaction at least once. These food-related incidents lead to around 30,000 visits to emergency rooms annually in the US.
According to DelveInsight’s Analysis, in the year 2022, the total prevalent cases of food allergy were approximately 57 million cases in the 7MM which are expected to increase by 2032. Based on etiology-specific factors, prevalent cases of food allergies were classified based on Cowmilk, Peanut, Eggs, Wheat, Tree nuts, Fish, Shellfish, Soy, and others in the United States with the majority of cases attributed by Shellfish.
Moreover, the prevalence of food allergy varies widely in different geographical locations, depending upon the influence of cultures on dietary habits. For example, peanut allergy is one of the most common causes of food anaphylaxis in the United States and the United Kingdom, but it is not as common in Italy and Spain, where peanut consumption is significantly lower.
The green light was given merely two months following the FDA’s acceptance of the companies’ additional biologics license application for this particular use. With Xolair injections scheduled every two to four weeks, the timing determined by both body weight and serum immunoglobulin E (IgE) levels, patients would enjoy a safeguard.
The FDA approval is rooted in encouraging findings from the Phase III OUtMATCH investigation, which examined Xolair’s effects in individuals aged 1 to 55 who have allergies to peanuts and a minimum of two other food allergens such as milk, egg, wheat, cashew, hazelnut, and walnut. The OUtMATCH research is supported and financed by the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH), and is currently underway through the NIAID-funded Consortium for Food Allergy Research (CoFAR) at 10 medical facilities nationwide, spearheaded by Johns Hopkins Children’s Center and jointly led by Stanford School of Medicine. Comprehensive findings from this study will be highlighted in a special session at the 2024 American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting on Sunday, February 25th.
Sung Poblete, R.N., Ph.D., the CEO of FARE (Food Allergy Research and Education), emphasized the urgency for a fresh strategy to prevent severe and sometimes life-threatening allergic reactions and emergencies, as the number of affected individuals continues to rise. Poblete, who has personal experience with food allergies, understands the profound effects they can have on individuals and their families. Poblete expressed solidarity with the community’s enthusiasm regarding this approval.
Patients in the OUtMATCH study initially couldn’t handle doses of up to 100 mg of peanut protein (roughly one-third of a peanut), as well as up to 300 mg each of milk, egg, and cashew protein. Following a 16 to 20-week period of treatment with either Xolair or a placebo, every participant underwent four food challenges. These challenges involved receiving increasing amounts of the allergenic foods (along with a placebo substance) to evaluate their ability to consume a single dose of at least 600 mg of peanut protein (the primary goal) and a single dose of at least 1,000 mg of milk, egg, or cashew protein (secondary goals) without encountering moderate to severe allergic reactions.
The findings of the food allergy clinical trial study revealed that a significantly greater number of patients (68%) who underwent treatment with Xolair for 16 to 20 weeks were able to tolerate at least 600 mg of peanut protein without experiencing moderate to severe allergic reactions, as opposed to 5% of those who received the placebo (p<0.0001). This quantity is approximately equal to two and a half peanuts or half a teaspoon of standard peanut butter.
Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America (AAFA), highlighted that the burden of coping with food allergies can be significant for individuals and their families. This challenge becomes especially apparent during occasions such as children’s birthday celebrations, school meal times, and festive gatherings with loved ones. With the increasing number of people affected by food allergies, the announcement of this development brings optimism for those looking for a new approach to better handle their food allergies.
Furthermore, a considerably higher percentage of patients who received Xolair, in comparison to those who were given a placebo, were able to tolerate at least 1,000 mg of protein from milk (66% vs. 11%; p<0.0001), egg (67% vs. 0%; p<0.0001), or cashew (42% vs. 3%; p<0.0001) without experiencing moderate to severe allergic reactions. This quantity roughly equals about two tablespoons of 1% milk, a quarter of an egg, or three and a half cashews. Although patients in the study were able to handle these amounts of food, it is advised to use Xolair in conjunction with continued avoidance of the specific food allergens.
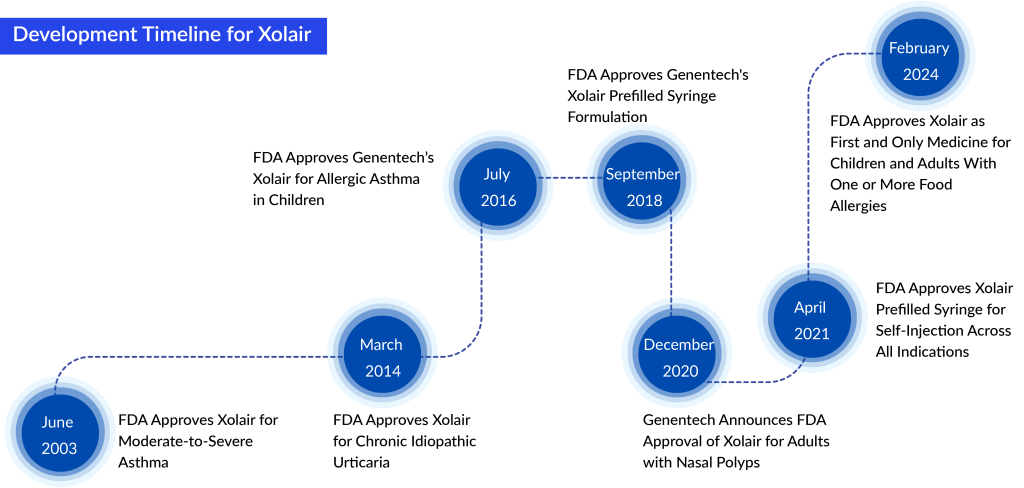
This is the fourth FDA-approved use for Xolair, covering various allergic and inflammatory conditions such as moderate to severe persistent allergic asthma, chronic spontaneous urticaria (CSU), and chronic rhinosinusitis with nasal polyps (CRSwNP). Since its first approval in 2003, over 700,000 patients in the US have received treatment with Xolair.
Xolair received its initial FDA approval in 2003 to treat asthma. Last year, sales of the medication amounted to $3.9 billion. Its mechanism involves blocking IgE antibodies, which are responsible for the chemical processes leading to the inflammation observed in allergy and asthma episodes. In 2018, the FDA recognized Xolair’s potential by granting it breakthrough status for addressing food allergies.
Last September, Nestle transferred ownership of its peanut allergy medication Palforzia to Stallergenes Greer for an undisclosed amount. Palforzia, which became the first FDA-approved peanut allergy drug in 2020, did not achieve the projected sales figures of $1.28 billion by 2024. Nestle had acquired Palforzia through a $2.1 billion acquisition of Aimmune Therapeutics.
Xolair, a prescribed biologic medication administered via subcutaneous injection, is uniquely formulated to inhibit IgE, a key factor in triggering food allergy responses, as approved by the FDA. The recommended dosage ranges from 75 mg to 600 mg, to be taken every 2 or 4 weeks, depending on factors such as total serum IgE levels and body weight. Administration of Xolair injections can be conducted by healthcare professionals either in a medical facility or by patients themselves at home following initial administration by a healthcare provider. The suitability for self-injection will be determined by healthcare practitioners based on individual patient circumstances.
Genentech and Novartis are dedicated to aiding individuals in obtaining the medications they need and providing extensive support for those prescribed Xolair, aiming to reduce obstacles to accessing and reimbursing the treatment. Genentech offers patient assistance programs through Genentech Access Solutions for eligible individuals. In the United States, Genentech collaborates with Novartis Pharmaceuticals Corporation in the development and joint promotion of Xolair.

Downloads
Article in PDF
Recent Articles
- GSK consolidates DT and TT vaccine, AZ gears up for FDA filing, Novartis comes up with flex prici...
- J&J ‘s COVID-19 vaccine; Roche, AC Immune’s anti-Tau Alzheimer’s drug; Sil...
- Artios collaborates with Novartis; Scholar Rock eyes Phase III trial; Novavax starts crossover ar...
- FDA Approves; AZ nabs; Nordisk settles; Novartis pays
- Colony Stimulating Factor 1 Receptor (CSF1R) and Its connection with Memory Retention



