X4 Pharmaceuticals’ XOLREMDI for WHIM Syndrome: First Targeted Treatment
May 10, 2024
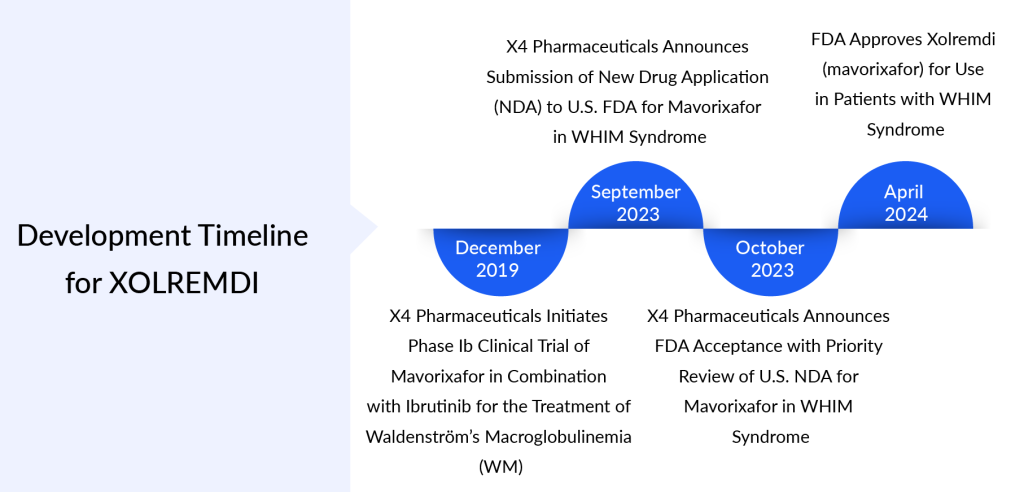
X4 Pharmaceuticals has gained approval for its first product, XOLREMDI (mavorixafor), marking its entrance into the commercial rare disease market. The FDA approved mavorixafor on April 29, 2024, specifically for patients aged 12 and above diagnosed with WHIM syndrome (characterized by warts, hypogammaglobulinemia, infections, and myelokathexis). XOLREMDI’s approval signifies the end of a ten-year voyage for X4, which emerged in 2015 after acquiring mavorixafor, previously identified as X4P-001, from Sanofi.
X4 has set the cost of XOLREMDI at approximately $496,000 annually for patients weighing over 50 kilograms, and roughly $372,000 for those weighing 50 kilograms or less. Additionally, upon FDA approval of XOLREMDI for WHIM syndrome treatment, X4 has been awarded a Rare Pediatric Disease Priority Review Voucher, which can be utilized for expedited review of future applications or traded to another pharmaceutical company.
WHIM syndrome is thin on the ground and indicative of its erroneous rarity; many healthcare professionals are unfamiliar with this condition. The extreme paucity of WHIMS and its analysis identifies a lack of awareness of the disease, eventually hampering proper demographic coverage to provide better insights, especially when it is part of a pool including defects of intrinsic and innate immunity sharing its nodes with other genetic conditions.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Nkarta’s Anti-CD19 Allogeneic CAR-NK Cell Therapy, NKX019; Eisai Presents Results of lecanemab fo...
- Which Pipeline Therapy Will Change the WHIM Syndrome Treatment Market Scenario?
- C-X-C Chemokine Receptor Inhibitors–The Emerging Therapeutic Agents
- X4 Pharmaceuticals’ XOLREMDI FDA Approval; ONO to Acquire Deciphera Pharmaceuticals; Johnson &...

Considering its genetic basis and the pivotal role of the CRCX4 gene in disease development, WHIMS on record is not restrained to the aforementioned mutation. Apart from the population statistics on record, accurately diagnosing WHIM syndrome remains a major challenge for physicians owing to the heterogeneity and non-specific nature of symptoms. Given its rarity and equally challenging diagnosis, WHIM is overall troublesome with trifling coverage in the medical literature.
There were 174 diagnosed prevalent cases of WHIM syndrome estimated to have occurred in the 7MM in 2022 of which 117 of the accounted cases were estimated to be from the US alone and these cases are anticipated to increase by 2032, as per DelveInsight.
XOLREMDI’s approval marks a significant milestone as it becomes the first medication sanctioned by the FDA for addressing WHIM. XOLREMDI is an orally administered inhibitor of CXCR4 intended to stimulate the movement of white blood cells, including neutrophils, lymphocytes, and monocytes, from the bone marrow to the bloodstream, to enhance immune function in cases of deficiency. The FDA awarded Breakthrough Therapy Designation to mavorixafor for WHIM syndrome and assessed the New Drug Application (NDA) with Priority Review, a classification reserved for treatments with promising potential to substantially enhance the management, diagnosis, or prevention of severe conditions.
“The approval of XOLREMDI marks a pivotal moment for both X4 and the WHIM syndrome community,” stated Paula Ragan, Ph.D., President and CEO of X4 Pharmaceuticals. “We extend our heartfelt gratitude to individuals affected by WHIM syndrome, their families, participating investigators, U.S. regulators for their ongoing dedication to rare disease therapies, and our committed staff for bringing this innovative treatment to fruition.”
Jorey Berry, President and CEO of the Immune Deficiency Foundation (IDF), emphasized the significance of innovative treatments for individuals with primary immunodeficiency. Berry highlighted the approval of XOLREMDI as a significant milestone for those with WHIM syndrome, a condition prone to severe infections. Berry expressed satisfaction in collaborating with X4 to deliver this vital treatment to the underserved rare disease community.
The approval of XOLREMDI by the FDA was based on findings from the crucial 4WHIM Phase III clinical trial. This trial, conducted globally, involved a randomized, double-blind, placebo-controlled study spanning 52 weeks across multiple centers. It aimed to assess the effectiveness and safety of XOLREMDI in individuals aged 12 and above diagnosed with WHIM syndrome. The effectiveness of XOLREMDI was evaluated through improvements in absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC), and a decrease in infections.
Results from the 4WHIM trial showed that treatment with XOLREMDI significantly increased the time during which ANC remained above certain thresholds (≥500 cells/microliter) compared to placebo (p<0.0001). Similarly, it also increased the time above the threshold for ALC (≥1000 cells/microliter) compared to placebo (p<0.0001). Further assessment of XOLREMDI’s efficacy was done through a combined endpoint, which included total infection score and total wart change score using a Win-Ratio method. Analysis of this composite endpoint revealed a roughly 40% decrease in total infection score, taking into account infection severity, in patients treated with XOLREMDI compared to those on placebo. However, there was no notable difference in total wart change scores between the XOLREMDI and placebo groups throughout the 52-week duration.
Moreover, WHIM syndrome treatment with XOLREMDI led to a significant 60% reduction in the annualized infection rate compared to patients treated with placebo. The most frequently reported adverse reactions in the 4WHIM trial, occurring in at least 10% of participants and more frequently than with placebo, included thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
Teresa K. Tarrant, M.D., who serves as an Associate Professor of Medicine specializing in Rheumatology and Immunology at Duke University School of Medicine, and is also a lead investigator in the 4WHIM trial, expressed her thoughts on the recent development. She stated, “Previously, care for individuals with WHIM syndrome primarily centered on managing symptoms rather than tackling the root cause of the condition — the malfunctioning CXCR4 pathway. The approval of XOLREMDI is an exciting milestone as it offers a targeted therapy aimed at restoring proper CXCR4 pathway function. This treatment has shown promising results in increasing absolute neutrophil and lymphocyte counts, thus enhancing WHIM patients’ ability to combat infections.”
In addition to WHIM syndrome, X4 is also experimenting with mavorixafor in chronic neutropenia, intending to initiate an advanced-stage study for this condition later this year. XOLREMDI may also get competition from the other pipeline therapies for WHIM syndrome. One such potential candidate in the WHIM syndrome pipeline includes the plerixafor of the National Institute of Allergy and Infectious Diseases (NIAID).
Plerixafor (Mozobil, AMD3100) is a US FDA-approved product that mobilizes CD34+ hematopoietic stem cells to the peripheral blood for collection (in combination with G-CSF) and subsequent autologous transplantation in adult patients with non-Hodgkin’s lymphoma and multiple myeloma. In March 2019, the EMA’s Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending label extension plerixafor for use in pediatric patients (1 to less than 18 years). The National Institute of Allergy and Infectious Diseases is developing plerixafor for the treatment of WHIM syndrome, and the drug has completed Phase III clinical trials.
It will be interesting to see how XOLREMDI retains its position in the WHIM syndrome treatment market as it offers a unique mechanism of action, potentially providing superior efficacy and safety compared to existing treatments.
With ongoing clinical trials demonstrating encouraging outcomes and the possibility of regulatory approval on the horizon, XOLREMDI holds the potential to become the standard of care for WHIM syndrome patients. Moreover, its targeted approach may pave the way for personalized medicine strategies, further enhancing its market potential. As awareness of WHIM syndrome grows and the demand for effective treatments increases, XOLREMDI stands ready to make a significant impact, offering hope to patients and healthcare providers alike.

Downloads
Article in PDF
Recent Articles
- C-X-C Chemokine Receptor Inhibitors–The Emerging Therapeutic Agents
- Nkarta’s Anti-CD19 Allogeneic CAR-NK Cell Therapy, NKX019; Eisai Presents Results of lecanemab fo...
- X4 Pharmaceuticals’ XOLREMDI FDA Approval; ONO to Acquire Deciphera Pharmaceuticals; Johnson &...
- Which Pipeline Therapy Will Change the WHIM Syndrome Treatment Market Scenario?