Ardelyx Overcomes Hurdles to Secure FDA Approval for Xphozah in Chronic Kidney Disease Treatment
Oct 23, 2023
Ardelyx has struck gold on its third attempt with Xphozah (tenapanor), the chronic kidney disease medication. Following two prior rejections, the FDA has granted its long-awaited approval. Xphozah, an innovative phosphate absorption inhibitor, is now officially sanctioned for the management of serum phosphate levels in adult chronic kidney disease patients undergoing dialysis, particularly those who cannot tolerate conventional phosphate binders or exhibit insufficient responses to such treatments.
The company estimates that the condition, known as hyperphosphatemia, impacts the majority of the 550K U.S. patients undergoing maintenance dialysis for chronic kidney disease. As per DelveInsight, the total diagnosed prevalent cases of CKD in the 7MM comprised of approximately 16 million cases in 2022 and are projected to increase during the forecasted period (2023-2032). CKD has been identified as a female-dominant disease; in our analysis, the number of females suffering was higher than males. In 2022, 52% of cases of CKD were in females, while 48% of cases were in males in the 7MM.
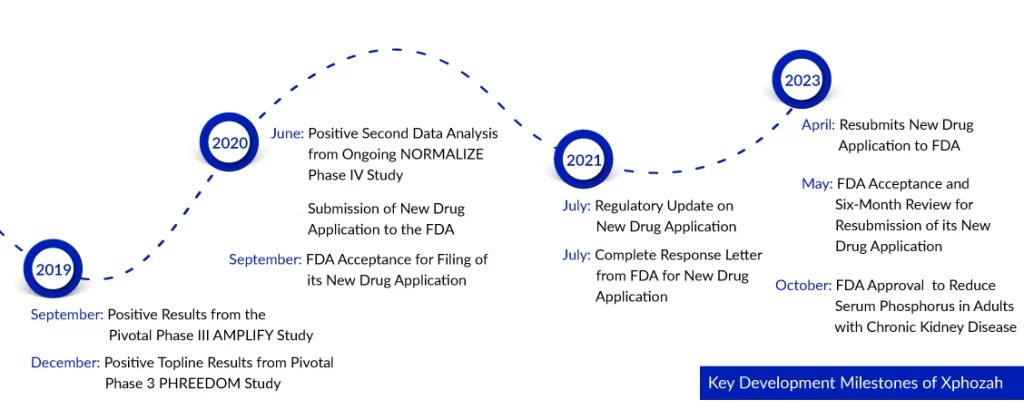
Ardelyx faced a challenging journey towards approval, marked by obstacles following the issuance of an FDA complete response letter in 2021. During that period, the agency raised concerns about the medication’s treatment effect, deeming it ‘small and of unclear clinical significance.’ But finally, on 17 Oct 2023, Ardelyx Inc. received FDA approval for XPHOZAH (tenapanor), a groundbreaking phosphate absorption inhibitor. This medication is designed to lower serum phosphorus levels in adults with chronic kidney disease who are on dialysis. It is intended as an additional treatment option for patients who do not respond well to phosphate binders or cannot tolerate them. XPHOZAH is taken twice daily as a single tablet and works through a unique mechanism that blocks the primary pathway of phosphate absorption.
Downloads
Article in PDF
Recent Articles
- Breakthroughs in Alport Syndrome Treatment: A New Era of Hope
- Recent Inclination of Pharma Companies, Emerging Pipeline Therapies Drive the IgA Nephropathy Mar...
- FDA Approves Teplizumab to Delay the Onset of Type 1 Diabetes; FDA Backs Ardelyx’s CKD Therapy Xp...
- Promising Nephrotic Syndrome Treatments: A Look into the Future
- Promising Data from the First Dedicated Kidney Outcomes Trial with GLP-1 Receptor Agonist, Semagl...

The approval of XPHOZAH marks a significant milestone for patients undergoing dialysis, their families, and the nephrology care community. It signifies a novel approach and a fresh treatment option for patients who, despite phosphate binder therapy, still grapple with elevated phosphorus levels. Ardelyx’s President and CEO, Mike Raab, emphasized, that this accomplishment reflects our unwavering commitment to the kidney community since our inception in 2007. This approval underscores the compelling clinical merits and potential benefits that XPHOZAH can offer to countless patients. This therapy’s journey began in our labs in 2008, and witnessing its availability for patients today is a testament to the relentless dedication, exceptional execution, and strong mission-driven ethos of the Ardelyx team. It is also a tribute to the patients, families, physicians, and clinical trial personnel who played a vital role in XPHOZAH’s development. The kidney community eagerly awaits the launch of XPHOZAH, and our world-class team is well-prepared with a pioneering product.
According to Dr. Glenn Chertow, a professor of medicine at Stanford University, he noted, “Managing hyperphosphatemia has consistently posed a clinical challenge, especially as the majority of patients undergoing maintenance dialysis struggle to consistently reach their target serum phosphate levels despite conventional phosphate binder treatment. XPHOZAH, unlike phosphate binders, functions as a phosphate absorption inhibitor. In cases where patients do not adequately respond to phosphate binders, XPHOZAH has demonstrated its ability to increase the number of patients achieving their target serum phosphate levels. I am confident that XPHOZAH can enhance the care provided to hyperphosphatemia patients, offering a new treatment option with a complementary mechanism of action.”
The FDA has granted approval for XPHOZAH for chronic kidney disease treatment following an extensive development program, which involved over 1,000 patients across three Phase III clinical trials. These trials assessed the effectiveness and safety of XPHOZAH as a standalone treatment and in conjunction with phosphate binder therapy. All three CKD trials successfully met their primary and key secondary endpoints, named PHREEDOM, BLOCK, and AMPLIFY. The data from these clinical trials demonstrates that XPHOZAH significantly reduces elevated serum phosphorus levels in patients undergoing maintenance hemodialysis.
Among patients with chronic kidney disease on dialysis who received XPHOZAH treatment in clinical trials, diarrhea emerged as the predominant adverse reaction, affecting 43 to 53 percent of individuals. Notably, most cases of diarrhea were mild to moderate in nature and showed improvement over time or with a reduction in dosage. While diarrhea was commonly observed shortly after initiating XPHOZAH treatment, it could manifest at any stage during the course of therapy. Severe diarrhea, though less common, was noted in approximately five percent of patients undergoing XPHOZAH treatment in these CKD trials. Ardelyx successfully concluded two open-label clinical trials (OPTIMIZE and NORMALIZE) aimed at assessing various strategies for incorporating XPHOZAH into clinical applications.
Apart from Ardelyx, several companies across the globe are also diligently working toward the development of novel treatment therapies with a considerable amount of success over the years. Key players, such as Aronora, Inc. (AB002), ProKidney (Renal Autologous Cell Therapy (REACT)), Boehringer Ingelheim/Eli Lilly and Company (JARDIANCE (empagliflozin)), KBP Biosciences (KBP-5074), Novo Nordisk A/S (Ziltivekimab), and others, are developing therapies for chronic kidney disease treatment. The anticipated launch of these chronic kidney disease therapies will give tough competition to Ardelyx’ Xphozah.
In the current treatment paradigm, the overall CKD treatment scope involves FARXIGA (SGLT2 inhibitor) which is used for diabetic and non-diabetic chronic kidney disease patients. FARXIGA captured a whooping market size of USD 111 million in 2019 and is expected to peak at USD 559 million by 2032.
Over recent years, top SGLT2 players have been in a race to rack up lucrative approvals. With Empagliflozin by Boehringer Ingelheim Pharmaceuticals, Inc., the chronic kidney disease treatment market can witness another approval with a medium uptake by 2023. Empagliflozin has collected landmark results with data from CKD trials and would be a competitor to FARXIGA as the data could help pave the way for a catch-up approval. With the label expansions, Farxiga’s sales have improved dramatically, but Jardiance is nowhere lagging. Based on the DelveInsight model, the therapy is expected to generate a chronic kidney disease treatment market size of USD 9 million by its launch year (2023), and this chronic kidney disease treatment market size is expected to increase and reach USD 772 million by 2032.
FAQs
Chronic kidney disease occurs when the kidneys get damaged and cannot filter blood as efficiently as they should. Chronic kidney disease is defined as abnormalities in kidney structure or function that have been present for more than three months and have a negative impact on health. Chronic kidney disease is categorized into five phases based on the extent of kidney damage and function. In stage 1, there is modest kidney disease, and in stage 5, the kidneys have stopped working. The severity of chronic kidney disease varies.
In the early stages of kidney disease, there are usually no chronic kidney disease symptoms. It can only be diagnosed if a blood or urine test is performed for another reason and the results show a kidney issue. Tiredness, swollen ankles, feet, or hands, shortness of breath, unwellness, and blood in the urine indicate a more advanced stage.
Chronic kidney disease can be detected using blood and urine tests. The eGFR blood test measures the efficiency with which the kidneys function. Ultrasound, CT scan, X-ray, and MRI imaging examinations are also used for chronic kidney disease diagnosis.
There is no cure for CKD, however, treatment can help ease symptoms and keep it from worsening. The treatment approach involves dietary and lifestyle adjustments, as well as medications to decrease blood pressure, blood sugar, or cholesterol. Diuretics are medications that help the kidneys excrete water and reduce edoema. In some circumstances, dialysis may be required. When the kidneys have failed, as in severe CKD, a kidney transplant may be an option.

Downloads
Article in PDF
Recent Articles
- AstraZeneca’s Imfinzi Shows Positive Results; Novartis Announces Results of Tislelizumab; FDA Gra...
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- World Kidney Day
- Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astel...
- B. Braun’s Introcan Safety 2 IV Catheter; Everly Health’s At-Home Collection Kidney Health Test; ...