YORVIPATH for Hypoparathyroidism Treatment – The First and Only Therapy for Therapy for Adults
Aug 16, 2024
A year after rejecting it, the FDA has now approved Ascendis Pharma’s once-daily injectable YORVIPATH for hypoparathyroidism treatment. Ascendis Pharma’s YORVIPATH (palopegteriparatide), formerly called TransCon PTH, has been approved for the treatment of adults with hypoparathyroidism. This rare condition, characterized by a lack of parathyroid hormone (PTH), impacts approximately 270K individuals in the seven major countries [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan], as per DelveInsight.
The company initially planned to launch the drug 15 months ago, but the FDA unexpectedly rejected it with a complete response letter (CRL). Three months ago, Ascendis was prepared to proceed again, but the US regulator requested an additional three months to make its decision. Consequently, YORVIPATH won’t be available right away.
Ascendis anticipates that the initial supply will be ready in the first quarter of next year, although there is a possibility of advancing the launch to the fourth quarter of this year if the FDA approves the commercialization of already manufactured doses. Manufacturing issues were a concern 15 months ago when the FDA highlighted problems with Ascendis’ production control strategy, citing variability in the doses delivered.
Downloads
Article in PDF
Recent Articles
- Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Ma...
- Hyperthyroidism Treatment Market and Drugs
- Tecelra by Adaptimmune: First FDA-Approved Engineered Cell Therapy for Solid Tumors; GSK’s ...
- FDA Approves Ascendis Pharma’s YORVIPATH; ARS Pharma Gets FDA Green Light for First Nasal Spray; ...
YORVIPATH is a prodrug of parathyroid hormone (PTH 1-34) administered once daily, designed to provide parathyroid hormone levels within the normal physiological range across the 24-hour dosing period. YORVIPATH was granted marketing authorization by the European Commission (EC) in November 2023 as a PTH replacement therapy indicated for the treatment of adults with chronic hypoparathyroidism. On January 31, 2024, Ascendis Pharma announced the launch of YORVIPATH in Germany.
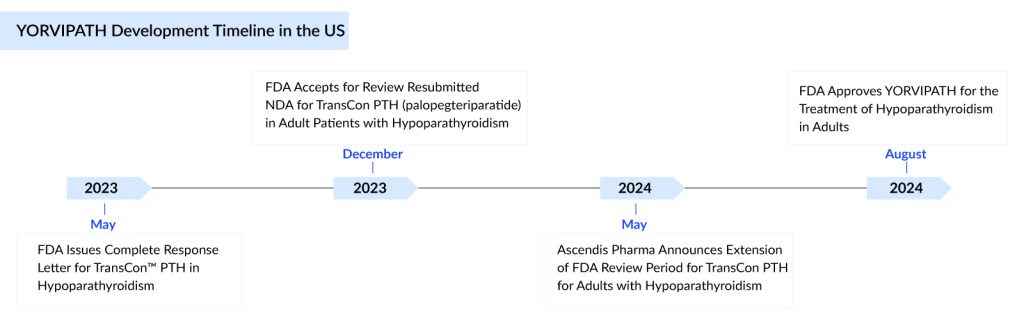
In April 2024, the United Kingdom’s Medicines & Healthcare Products Regulatory Agency (MHRA) granted marketing authorization for YORVIPATH in Great Britain as a PTH replacement therapy indicated for the treatment of adults with chronic hypoparathyroidism and has also granted YORVIPATH orphan drug status.
The FDA approved YORVIPATH for hypoparathyroidism treatment after examining the clinical data for TransCon PTH (palopegteriparatide) provided in the Ascendis’ New Drug Application, which included results from the global Phase II PaTH Forward and Phase III PaTHway studies.
On August 12, Ascendis Pharma’s shares rose in pre-market trading following the approval, increasing by 11% to $141. Over the last year, the stock has surged by 38%. YORVIPATH is Ascendis’ second product following SKYTROKA, a long-acting growth hormone for children that was approved in 2021. In the previous year, SKYTROKA generated sales of 179 million euros ($194 million). Ascendis anticipates that SKYTROKA sales will range between 320 million euros ($349 million) and 340 million euros ($371 million) this year.
The FDA’s approval of Ascendis Pharma’s YORVIPATH comes at a crucial moment for hypoparathyroidism patients, as Takeda Pharmaceutical is about to discontinue the only existing hypoparathyroidism drug for this rare condition. With Takeda’s hypoparathyroidism drug expected to be unavailable by year’s end, patients have been eagerly awaiting an alternative, and the timely FDA approval of Ascendis Pharma’s hypoparathyroidism drug provides just that, though later than anticipated.
Eli Lilly’s osteoporosis treatment FORTEO (teriparatide), a PTH analog, is sometimes used off-label for patients with severe hypoparathyroidism. However, a new FDA-approved hypoparathyroidism treatment will address the impending shortage of options for these patients.
Several other companies also have hypoparathyroidism drugs in the pipeline. Ascendis’s closet rival could be Amolyt Pharma’s Eneboparatide, currently in Phase III trials with a peptide aimed at targeting the parathyroid hormone receptor. Initial data is anticipated in 2025.
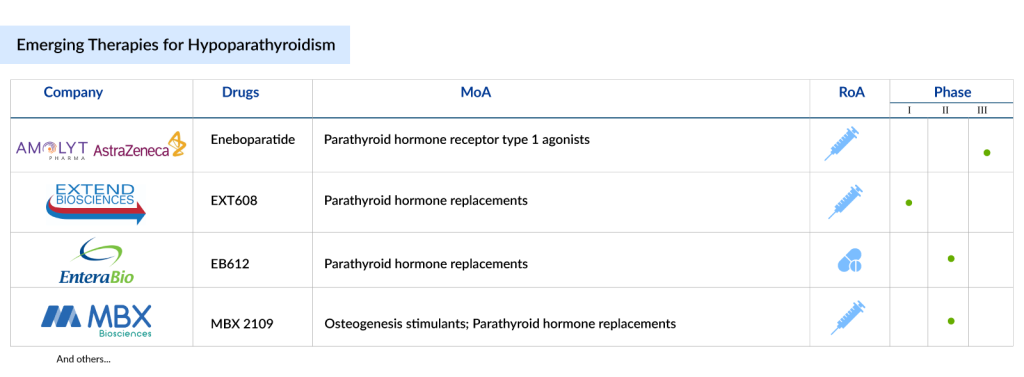
Phase II data showed that eneboparatide achieved normalization of serum calcium levels as well as the potential to eliminate dependence on daily calcium and vitamin D supplementation. In adults with chronic hypoparathyroidism and hypercalciuria, results showed that eneboparatide normalized calcium in urine. In addition, for patients with hypoparathyroidism, eneboparatide preserved bone mineral density, an important potential benefit in patients with an increased risk of osteopenia or osteoporosis. Earlier this year, AstraZeneca purchased Amolyt for $800 million upfront.
Extend Biosciences’ EXT608 is currently in the early stages of clinical development. Developed using ExtendBio’s D-VITylation platform, the compound is fully active at the PTH receptor upon injection, and the company plans to make it available for patients in a simple, easy-to-use single-use disposable syringe.
An interim data analysis showed that EXT608 was safe and well tolerated, with no serious adverse events reported. It also showed a dose-dependent increase in serum calcium, without a concomitant increase in urinary calcium. EnteraBio is currently testing an oral formulation of PTH. EnteraBio’s EB612 is in the early stages of clinical trials for hypoparathyroidism treatment.
While MBX Biosciences’ MBX 2109 is in Phase II hypoparathyroidism clinical trials. Septerna Therapeutics, a startup based in South San Francisco, is pursuing a different strategy with an oral small molecule aimed at targeting and activating the parathyroid hormone 1 receptor. Just over a year ago, Septerna secured $150 million in a Series B funding round to help advance its hypoparathyroidism treatment drug into clinical trials.
The anticipated launch of these hypoparathyroidism emerging therapies poses a significant threat to Ascendis Pharma’s YORVIPATH, primarily due to their innovative mechanisms of action and potentially superior efficacy profiles. These hypoparathyroidism therapies often come with cutting-edge technology and novel approaches that could address the same medical needs as YORVIPATH but with added benefits such as fewer side effects or more convenient administration.
As competitors continue to advance their research and development, they may introduce products that not only rival YORVIPATH in terms of effectiveness but also potentially offer differentiated benefits, making it challenging for YORVIPATH to maintain its hypoparathyroidism market dominance.
Furthermore, the rapid pace of innovation in the pharmaceutical industry means that new entrants can quickly capture hypoparathyroidism treatment market share if they present compelling evidence of their products’ advantages. Competitive therapies that achieve faster regulatory approvals or demonstrate robust clinical outcomes could swiftly attract both healthcare providers and patients, thereby diminishing YORVIPATH’s market presence. The continuous evolution of hypoparathyroidism treatment options requires Ascendis Pharma to stay agile, enhance its product offerings, and strengthen its value proposition to fend off competitive pressures effectively.

Downloads
Article in PDF
Recent Articles
- FDA Approves Ascendis Pharma’s YORVIPATH; ARS Pharma Gets FDA Green Light for First Nasal Spray; ...
- Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Ma...
- Hyperthyroidism Treatment Market and Drugs
- Tecelra by Adaptimmune: First FDA-Approved Engineered Cell Therapy for Solid Tumors; GSK’s ...



